- Submissions

Full Text
Modern Research in Dentistry
A Review on Natural Resources and Method of Hydroxyapatite Synthesis for Biomedical Application
Luddin N1*, Abd Hamid IF1, Mohd Nordin NN1 and Nurddin SMASM2
1School of Dental Sciences, Universiti Sains Malaysia, Health Campus, 16150 Kubang Kerian, Kelantan, Malaysia
2Mineral Research Centre, Jalan Sultan Azlan Shah, 31400 Ipoh, Perak, Malaysia
*Corresponding author: Norhayati Luddin, School of Dental Sciences, Universiti Sains Malaysia, Health Campus, 16150 Kubang Kerian, Kelantan, Malaysia
Submission: July 27, 2023;Published: August 22, 2023

ISSN:2637-7764Volume8 Issue1
Abstract
Hydroxyapatite (HA) is one of the most studied biomaterials in medical and dental fields as it is the main component of bone and teeth. It can be derived from several sources using different methods of synthesis. This review provides an insight into natural resources for HA and methods that are available for its synthesis.
Keywords: Hydroxyapatite; Natural sources; Synthesis; Limestone; Dental biomaterial
Introduction
Biomaterial is a substance that refers to any natural or synthetic material that is replaced in the human body for medical purposes. It performs the same role as biological material in assessing, sustaining, or replacing any tissue or organ [1]. Hydroxyapatite (Ca10(PO4)6(OH)2) is a biomaterial that can be found in nature and also the main mineral component for teeth and bones [2]. Hydroxyapatite (HA) is one of the biomaterial compounds that has been regarded as non-toxic, non-inflammatory and non-immunogenic agent which can be directly formed as a chemical bond with living tissues [3]. This bioactive HA is thermodynamically stable in its crystalline state in a body fluid and thus poses a wide range of applications in the biomedical field of dentistry and orthopaedics [4]. It is used as an implantable material in dentistry, maxillofacial and orthopaedic surgery for repairing bone defects and as a coating material for metallic implants. Hydroxyapatite consists of calcium phosphate that promotes osseointegration and a new bone formation process due to their uniqueness properties which has similarity to mineral bones [5].
Basically, HA has the molecular weight of 1004.6g/mol and it consists of calcium orthophosphate where the calcium element can be obtained from calcium hydroxide (Ca(OH)2 while the orthophosphate groups can be obtained from phosphoric acid (H3PO4) [6]. In comparison, to other calcium phosphate (Ca/P) family, the HA has a molar ratio 1.67 [7]. Natural HA is non-stoichiometric because the calcium and phosphorus from natural sources differ depending on its original sources. In some cases, like extraction of HA from mammalian and equatic sources, it may also provide some trace elements such as magnesium ion (Mg2+), ferrous ion (Fe2+), sodium (Na) and potassium (K+). Even in a small amount, these trace elements play a role in speeding up the formation process of bone and teeth regeneration [1,8].
Natural Sources of Hydroxyapatite
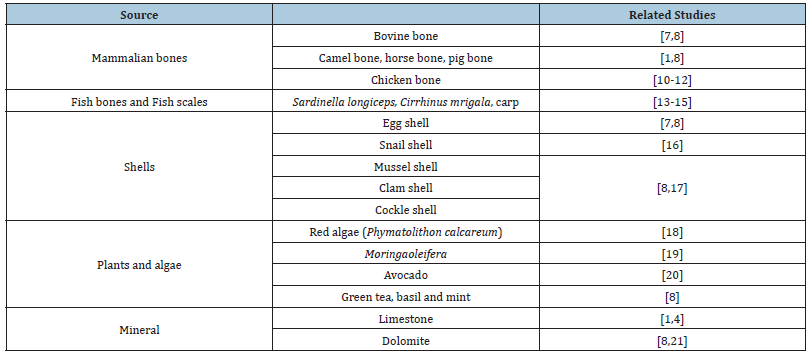
Hydroxyapatite (HA) can be derived from both synthetic and natural sources. Natural HA can be extracted from biological sources available around nature. Some researchers have successfully extracted the natural sources of HA from mammalian bones, chicken bones, fish bones and scales, shell-based material from aquatic shells and eggshells, plants (stalk, leaves, flower, fruit peel), algae as well as from mineral sources (Table 1). Palmer et al. [9] mentioned that the HA prepared from natural sources such as from bones, eggshells and seashells can demonstrate better biological properties due to the presence of beneficial cations like Na+, K+, Mg2+, Sr2+, Zn2+, and Al3+ or anions like F-, Cl-, SO4 2-, and CO32 or the presence of both anion and cation. The HA from these natural sources together with these trace minerals are proven in speeding up some medical application such as in rapid bone regeneration.
Table 1:Natural source for HA extraction.
Mammalian bones
Additionally, mammalian bones from bovine, camel, horse and pig are sources of HA [8]. Bovine bone mostly had been used to extract the HA because of meat demand. For this, more bovine source has been used compared to other animals because their abundance will be wasted if these bones were not used. Interestingly, part of the femoral bone from bovine is usually chosen because this part is similar to human bone structurally and morphologically [1]. Bovine bone contains calcium phosphate up to 58.3%. Thus, the derivation of HA from bovine bone was preferred in synthesizing HA since their main compounds were calcium and phosphate [7].
Before synthesizing HA from mammalian bones, usually pretreatment like boiling, washing with acid, alkali, acid-alkali solution is necessary to be carried out to remove dirt, fats, protein and soft tissues. Sometimes, surfactants were used in a pretreatment of bones to remove soft tissues. Calcination and combination with other methods are preferred in synthesizing HA from mammalian bones. The thermal process from the calcination method removes the organic constituent in the bones as well as remove pathogens that can relate to the spread of diseases such as bovine spongiform encephalopathy and Creutzfeldt Jakob disease [8].
Apart from that, biowaste of chicken bones are also good source of HA. The chicken bones consist of both organic and inorganic materials that have abundance of minerals especially calcium and phosphate where the chicken bone particles are normally composed approximately about 65% of mineral HA, 25% of protein and 10% of water [1,10,11]. Similar to mammalian bones, calcination method was preferred in chicken femur bones waste to produce HA. As Rajesh et al. [10] suggested that calcination process was conducted between 600 to 1000 ℃, Bee & Abdul Hamid [12] also ran a calcination method for chicken bone femur with different calcination temperature between 600 to 1000 ℃ to completely remove organic matrix from raw chicken bone. Upon calcination, the colour of chicken bones varies depending on the temperature of calcination from light grey, off-white and white powder. However, the HA originated from bones is still costly and high-risk infection can be transmitted from bones to the human body when implanted especially when the calcination process is not fully completed. Moreover, mammalian bones such as pig are often questionable because it is related to religious feeling [1].
Fish bones and fish scales
Similar to other bones, a marine source such as fish bones and fish scales were commonly selected as one of natural source of HA. Among the reasons for their popular usage is abundance of marine waste accumulated if not used. As the consumption of fish significantly increased, the fish waste production in the form of scales and bones also increased. Therefore, this type of HA extraction has been one of the popular methods. Moreover, fish bones are rich in calcium, phosphate and carbonate that comprise the main source of HA [8]. In addition, the HA from fish bones and fish scales exhibits greater biological activity due to the presence of all cations (such as Mg2+, Al3+, Sr2+, Zn2+, K+ Na+) and all anions (such as Cl- and F-) that will boost the effectiveness in a variety of biomedical applications, such as accelerating bone repair [1].
Shells
The other source of raw material of HA is from shells where the shells are rich in calcium carbonate (CaCO3) [7]. The shells available in nature include cockle shells, clamshells, mussel shells, crab shells, snail shells, and eggshells. Mostly, all shells contain about 94-97% calcium carbonate (CaCO3). As for the eggshells, they contain about 94% carbonate (CaCO3). As such, eggshells have been used to synthesize HA due to their ease of availability and high in calcium content [8]. Generally, the calcium derived from carbonate (CaCO3) is normally used for the synthesis of HA. As such, these practices are not only important in the production of HA but may reduce environmental pollution as well as industrial cost [7,8]. However, some consideration should be made especially when synthesizing HA from eggshells because there is a risk for infection from Salmonella enteritidis. These bacteria may have transmitted viruses originating from eggshells into the surrounding tissues if the HA is derived from infected eggshells implanted in a human body [1,13-17].
Plants and algae
Previous studies reported that plants and algae have the potential to become a source of HA [18-20]. The HA had been extracted from plants parts such as from leaves, stalk, flower, fruit peel and some researchers have successfully extracted HA from algae [8]. Plants that are rich in carbonate (CaCO3) could be the sources for HA including red algae (Phymatolithon calcareum), Moringaoleifera leaves, avocado fruit peel, leaves and stems of green tea, basil and mint [18-20]. The synthesis process in transforming Moringaoleifera leaves into HA is considered cost effective as it is low cost, a safe process and other than that, the materials are abundantly available. As such, it displays a good application in future tissue repair engineering [19]. Besides, red algae contain sufficiently porous interconnected CaCO3 with high similarity to human bones where the calcite produced from calcination of the red algae converts into HA and retains its naturally porous nature using a mixture of Diammonium Hydrogen Phosphate (DAHP) and Magnesium Nitrate (MgNO3) [18].
Mineral
Limestone is one of the natural minerals sources that has been used to synthesize HA. Naturally, limestone formation consists of precipitation of the animal shells or skeletons, foraminifera or algae that contained carbonate (CaCO3) [8]. The content of carbonate (CaCO3) in limestone is around 95% and it should be purified to produce calcium. Research on limestone has been popular because the synthesis of HA from limestone is regarded as an alternative to produce HA for application in dentistry and orthopedics. This is due to its close natural properties of teeth and bones [4,21]. However, instead of CaCO3, dolomite is widely present in sedimentary rocks of limestone. CaCO3 can be extracted from limestone and dolomite by purifying the CaCO3. These minerals are usually related to impurities that may affect the calcination process. Therefore, screening for natural sources of HA is important and the selection of HA synthesis are crucial because the physicochemical properties of synthesized HA such as Ca/P ratio, crystallinity, crystallites size and morphology can affect the properties of the obtained HA [1].
Additionally, trace minerals such as Magnesium (Mg) are crucial in any application of HA to the human body. Magnesium (Mg) from dolomite contributes to the mineralization of calcified tissue and thus stimulates the growth of bone tissue. This trace mineral is necessary for the growth of bones and metabolic activity. Additionally, this Mg component included in the HA structure aids in promoting osteoblast proliferation, which is crucial for bone production [1].
Synthesis of Hydroxyapatite
Hydroxyapatite can be synthesized either through chemical or natural synthesis by using natural calcium sources. Chemical synthesis of HA may control the morphology, crystallinity and stoichiometric composition (1.67) of the HA, but this chemical synthesis is costly, complicated and time consuming that mostly produces undesirable by-products after synthesis [1]. Over the decades, there have been several methods to synthesize HA from natural sources. Generally, it can be classified into dry methods, wet methods and high temperature methods [22]. But in some approaches, synthesis of HA from natural sources can be specified into dry methods, wet methods, hydrothermal methods, high temperature processes, synthesis by biogenic sources and mixture of combination methods [2,4]. Each method has its own advantages and limitations in producing HA. As such, method of HA synthesis should be considered crucial when choosing the suitable methods for each different natural sources of HA.
Dry methods can be classified into solid-state and mechanochemical reaction where the precursor of chemicals (calcium and phosphate) is used in a dry form. Compared to the dry methods, wet methods used aqueous solution during synthesis of HA. Some examples of HA synthesis by wet methods are chemical precipitation, hydrothermal and hydrolysis. As for high temperature methods, high temperature is used to decompose the material in order to extract HA. There are two methods that can be selected for this category which are combustion and pyrolysis methods. However, through the journey in producing HA from natural sources, these approaches were rarely used due to the poor control over processing parameter as well as the production of secondary aggregates [22].
Dry methods
Chemical precursors in dry methods for both solid-state and mechanochemical methods were calcium and phosphate. These precursors are in a dry form and the process of synthesizing HA does not require the use of a solvent [4,22].
Solid-state: Method of solid-state is a simple method where calcium and phosphate are milled and calcined to obtain HA. As the reactants used are in a solid form, thermal treatment with high temperature is necessary to initiate solid-state ionic diffusion of reagents [23]. For Example, Pramanik et al. [24] produced α-tri calcium phosphate (α-TCP) after sintering at 1250 ℃. Meanwhile, Koonawoot et al. [25] produced a mixture of HA, TCP and calcium oxide where this process produced the highest composition of HA at a calcination temperature of 1250 ℃ for 2h.
Mechanochemical: Mechanochemical method is a method that employs compression, shear, or friction via grinding and milling to induce a chemical transformation [22]. Generally, mechanochemical methods use ball-milling or planetary mills at certain speeds or frequencies. This technique is conducted in a sealed vessel or jar made from materials such as stainless steel, agate or zirconia [26]. In mechanochemical approach, parameters like the rotational speed, milling time and mass ratio of the powder and ball should be investigated to produce the HA [27]. Compared to solid-state, the mechanochemical method produces a heterogeneous particle with an irregular shape. This is because of the perturbation of the surface-bonded species as a result of pressure, enhancing thermodynamic and kinetic reactions between solids [28]. Extraction of HA powder from eggshells powders were investigated via ball milling at various milling times followed by heat treatment. The eggshells were cleaned, dried and crushed with an agate mortar before it is mixed with Dehydrated Dicalcium Phosphate (DCPD) at a ratio of 4:3 together with deionized water. This mixture was then milled ranged from 1 hour to 10 hours in a planetary ball-mill at 170rpm. After milling, the slurry was dried at 150 ℃ for 24h followed by heating the dried powder to 1000 ℃ at a rate of 10 ℃/min for 1 h to finally producing the powdered HA [29]. This mechanochemical approach is considered as a simple, efficient and inexpensive method that produced HA in a powder form in a well-defined structure [30].
Both solid-state and mechanochemical methods are preferred for mass production of HA powders without worrying about the processing parameters because no precise and no control conditions are needed when dealing with these dry methods [4,27].
Wet methods
Wet method for HA synthesis can be divided into 3, chemical precipitation, hydrothermal and hydrolysis methods.
Chemical Precipitation: The most preferable approach in synthesizing HA via wet methods are chemical precipitation method. The chemical precipitation method is one of the broadest research techniques in synthesizing HA [21]. The other names for chemical precipitation method we had known as precipitation or precipitation aqueous because this method uses aqueous solution during synthesis of HA. Moreover, HA can be produced in relatively large quantities with the absence of the use of organic solvents at a reasonable cost and thus this chemical precipitation is in demand [31]. Usually, chemical precipitation methods must undergo several processes. The precursors of calcium and phosphate should be mixed according to the molar ratio of HA. Then the pH of this mixture should be adjusted to a specific alkaline pH and temperature also should be adjusted to range from room temperature to boiling point of water. After that, the solution is stirred for some time for ageing purposes, and then the precipitation solution is finally washed, filtered, and dried before crushing it into a powder form of HA [22]. The process of extracting HA using limestone as the raw material was studied using precipitation method where calcium oxide from limestone was mixed with diammonium hydrogen phosphate. The mixture was then washed, dried and crushed to remove impurities and homogenize the size of limestone. Finally, calcination process was proceeded at 900 ℃ for 4hours to extract the HA from limestone [32]. The chemical precipitation approach needed a few crucial processing parameters such as suitable pH and temperature for precipitation to occur and the right molar ratio of HA should be determined because the unbalanced molar ratio in the chemical precursor of calcium and phosphate could result in the formation of another compound [4,22].
Hydrothermal: Hydrothermal synthesis of HA is similar to the chemical precipitation method where the reaction method is in aqueous media but with variations in pressure and temperature [27]. As the hydrothermal time and temperature increased, the phase purity and Ca/P ratio improved, and the production of HA is in a better structure with increased crystallite size and crystallinity compared to low reaction temperature [1]. An autoclave or pressure vessel was usually used to create high temperature and high-pressure environments for this hydrothermal approach. This high pressure and high temperature will increase the reactivity, and the effect of the condensation creates the chemical bonds and forms a nucleus that ensures the production of a relatively stoichiometric and highly crystalline HA [33]. This approach was taken to investigate the HA synthesis by mixing calcium nitrate tetrahydrate and diammonium hydrogen phosphate at different temperatures for 3h. At pH 10, pure crystalline HA was generated and XRD patterns showed sharper peaks as temperature increased. But the HA produced were not consistent in shape and sizes. Thus, surfactants should be added to control the HA morphology [34]. Production of HA by hydrothermal method produces HA with higher crystallity compared to chemical precipitation but modifier such as surfactant should be included to generate controllable size and morphology of HA [22].
Hydrolysis: Hydrolysis method is a method that deals with water ionization process that causes the diffusion of hydrogen and hydroxide ions. This method basically hydrolyzed calcium phosphate with the produced formation of a non-stoichiometric HA. Synthesis parameters such as temperature, reaction duration, ratio and composition of the starting reagents, and pH in hydrolysis reaction may produce a better composition and morphology of synthesized HA in a crystal form [35]. Water and/or other organic solvents are used to hydrolyze HA from natural sources. For example, water was used in the hydrolysis of α-TCP to produce Calcium-Deficient Hydroxyapatite (CDHA) [36]. The α-TCP was also used to generate HA where the temperature of α-TCP suspension and water were maintained at certain temperature (20, 40, 60 and 100 ℃). When the temperature is maintained at 40 ℃, it takes 48h to completely synthesize HA by this hydrolysis process. Their findings revealed that when maintaining the temperature at 100 ℃, it only took 3h to produce HA [35]. Organic solvents such as alcohol at different concentrations were used in the study of hydrolysis of Calcium Hydrogen Phosphate Dehydrate (DCPD) into HA. But the alcohol concentration affected the crystallite size of HA. At the concentration of 0 to 70% alcohol, the crystallite size of HA decreased and at the concentration of 70 to 90%, the crystallite size of HA increased [37]. Hydrolysis method can produce HA with high purity but parameters such as time required to hydrolyze HA and appropriate concentration of solvents should be optimized to generate this pure HA.
High temperature methods
High temperature method is a process that promotes decomposition and reactions of HA precursors through firing cycles. This approach consists of two methods which are combustion and pyrolysis [4]. Both combustion and pyrolysis are rarely used in HA synthesis because of poor control over the processing parameter as well as the other production of secondary aggregates [22].
Combustion: Combustion method is carried out in a single step to obtain high purity of HA. This process is fast, low energy and exothermic which once combustion process started, it does not require any external heat source and enough with only a selfsustained chemical reaction occurs between an oxidant and organic fuel such as hydrazine or citric acid in an aqueous medium [4,22].
Pyrolysis: Pyrolysis is a high temperature method that involves spraying of a precursor into a hot zone of the electric furnace using ultrasonic generator. This spray approach production of final products of HA are formed through an aerosol process that atomizes a solution and heats the droplets to produce solid particles with no addition of fuel [4]. The reaction between vapors and gases at high temperature produced final powder product but the product is aggregated and agglomerated [27]. Limited studies have reported the synthesis of HA via pyrolysis method.
Conclusion
Hydroxyapatite can be obtained either synthetically or naturally. Among the natural resources from which the HA can be obtained are mammalian bones, fish bones and scales, shells, plants and algae and from mineral resources. Researchers need to choose the right resources to obtain the HA as some resources have limitations and disadvantages for biomedical purposes. Similarly, several methods can be used to synthesize HA from natural resources. However, we need to choose the right method as specific parameters are deemed crucial to ensure that the materials can be transformed into value added materials to be used for biomedical applications.
Acknowledgement
This work was supported by Universiti Sains Malaysia (USM): Research University Individual Grant (RUI)-1001/PPSG/8012355.
References
- Arokiasamy P, Abdullah MAB, Abd Rahim SZ, Luhar S, Sandu AV, et al. (2022) Synthesis methods of hydroxyapatite from natural sources: a review. Ceramics International 48(11): 14959-14979.
- Balhuc S, Campian R, Labunet A, Negucioiu M, Buduru S, et al. (2021) Dental applications of systems based on hydroxyapatite nanoparticles-an evidence-based update. Crystals 11(6): 674.
- Sobczak A, Kowalski Z, Wzorek Z (2009) Preparation of hydroxyapatite from animal bones. Acta of Bioengineering and Biomechanics 11(4): 23-28.
- Gomes D, Santos AMC, Neves GA, Menezes RR (2019) A brief review on hydroxyapatite production and use in biomedicine. Cerâmica 65(2019): 282-302.
- Siddiqui HA, Pickering KL, Mucalo MR (2018) A Review on the use of hydroxyapatite- carbonaceous structure composite in bone replacement materials for strengthening purposes. Materials 11(10): 1813.
- Arif S, Hermana GN, Khalida Z, Arif SMW, Puspita I (2020) Synthesis of biomaterial hydroxyapatite from limestone by using two-step conversion. International Journal of Science, Engineering and Information Technology 5(1): 236-238.
- Afriani F, Siswoyo, Amelia R, Hudatwi M, Zaitun, et al. (2020) Hydroxyapatite from natural sources: methods and its characteristics. IOP Conference Series: Earth and Environmental Science 599(2020): 012055.
- Mohd Pu'ad NAS, Koshy P, Abdullah HZ, Idris MI, Lee TC (2019) Syntheses of hydroxyapatite from natural sources. Heliyon 5(5): e01588.
- Palmer LC, Newcomb CJ, Kaltz SR, Spoerke ED, Stupp S (2008) Biomimetic systems for hydroxyapatite mineralization inspired by bone and enamel. Chemical Reviews 108(11): 4754-4783.
- Rajesh R, Hariharasubramanian A, Ravichandran YD (2012) Chicken bone as bioresource for bioceramic (hydroxyapatite). Phosphorus, Sulfur, and Silicon and the Related Elements 187(8): 914-925.
- Hanny, A, Islam MR, Sumdani MG, Rashidi NM (2019) The effects of sintering on the properties of epoxy composites reinforced with chicken bone-based hydroxyapatites. Polymer Testing 78: 105987.
- Bee SL, Abdul Hamid ZA (2019) Characterization of chicken bone waste-derived hydroxyapatite and its functionality on chitosan membrane for guided bone regeneration. Composites Part B: Engineering 163: 562-573.
- Bahraminasab M, Doostmohammadi N, Alizadeh A (2021) Low-cost synthesis of nano-hydroxyapatite from carp bone waste: Effect of calcination time and temperature. International Journal of Applied Ceramic Technology 18(3): 573-582.
- Sathiskumar S, Vanaraj S, Sabarinathan D, Bharath S, Sivarasan G, et al. (2019) Green synthesis of biocompatible nanostructured hydroxyapatite from Cirrhinus mrigala fish scale - A biowaste to biomaterial. Ceramics International 45(6): 7804-7810.
- Surya P, Nithin A, Sundaramanickam A, Sathish M (2021) Synthesis and characterization of nano-hydroxyapatite from Sardinella longiceps fish bone and its effects on human osteoblast bone cells. Journal of the Mechanical Behavior of Biomedical Materials 119: 104501.
- Charlena, Suparto IH, Putri DK (2015) Synthesis of hydroxyapatite from rice fields snail shell (Bellamya javanica) through wet method and pore modification using chitosan. Procedia Chemistry 17: 27-35.
- Pal A, Maity S, Chabri S, Bera S, Chowdhury AR, et al. (2016) Mechanochemical synthesis of nanocrystalline hydroxyapatite from Mercenaria clam shells and phosphoric acid. Biomedical Physics & Engineering Express 3(1): 015010.
- Agbeboh NI, Oladele IO, Daramola OO, Adediran AA, Olasukanmi OO, et al. (2020) Environmentally sustainable processes for the synthesis of hydroxyapatite. Heliyon 6(4): e03765.
- Govindaraj D, Rajan M (2016) Synthesis and spectral characterization of novel nano-hydroxyapatite from moringaoleifera Materials Today: Proceedings 3(6): 2394-2498.
- Pawar S, Theodore T, Hiremath PG (2019) Synthesis of hydroxyapatite from avocado fruit peel and its application for hexavalent chromium removal from aqueous solutions-adsorption isotherms and kinetics study. Rasayan Journal of Chemistry 12(4): 1964-1972.
- Sirait M, Sinulingga K, Siregar N, Siregar RSD (2020) Synthesis of hydroxyapatite from limestone by using precipitation method. Journal of Physics. Conference Series 1462(2020): 012058.
- Mohd Pu'ad NAS, Abdul Haq, RH, Mohd Noh, Abdullah HZ, Idris MI, et al. (2020) Syntheses Method of Hydroxyapatite: A Review. Materials Today: Proceedings 29(Part-1): 233-239.
- Cox SC, Walton RI, Mallick KK (2015) Comparison of techniques for the synthesis of hydroxyapatite. Bioinspired, Biomimetic and Nanobiomaterials 4(1): 37-47.
- Pramanik S, Agarwal AK, Rai KN, Garg A (2007) Development of high strength hydroxyapatite by solid-state-sintering process. Ceramics International 33(3): 419-426.
- Koonawoot R, Saelee C, Thiansem S, Punyanitya S (2012) Synthesis control and characterization of hydroxyapatite ceramic using a solid-state reaction. Biomaterial Symposium II.
- Achar TK, Bose A, Mal P, Beilstein J (2017) Mechanochemical synthesis of small organic molecules. Beilstein Journal of Organic Chemistry 13: 1907-1931.
- Sadat-Shojai M, Khorasani MT, Dinpanah-Khoshdargi E, Jamshidi A (2013) Synthesis methods for nanosized hydroxyapatite with diverse structures. Acta Biomateriala 9(8): 7591-7621.
- Mochales C, Wilson RM, Dowker SEP, Ginebra MP (2011) Dry mechanosynthesis of nanocrystalline calcium deficient hydroxyapatite: structural characterisation. Journal of Alloys and Compounds 509(27): 7389-7394.
- Wu SC, Hsu HC, Hsu SK, Chang YC, Ho WF (2016) Synthesis of hydroxyapatite from eggshell powders through ball milling and heat treatment. Journal of Asian Ceramic Societies 4(1): 85-90.
- Fahami A, Ebrahimi-Kahrizsangi R, Nasiri-Tabrizi B (2011) Mechanochemical synthesis of hydroxyapatite/titanium nanocomposite. Solid State Sciences 13(1): 135-141.
- Nayak AK (2010) Hyrdroxyapatite synthesis methodologies: an overview. International Journal of ChemTech Research 2(2): 903-907.
- Habibie S, Wargadipura AHS, Gustiono D, Herdianto N, Riswoko A, et al. (2017) Production and characterization of hydroxyapatite bone substitute material performed from Indonesian limestone. International Journal of Biomedical Engineering and Science 4(1): 11-23.
- Fihri A, Len C, Varma RS, Solhy A (2017) Hydroxyapatite: a review of syntheses, structure and applications in heterogeneous catalysis. Coordination Chemistry Reviews 347: 48-76.
- Nagata, F, Yamauchi Y, Tomita M, Kato K (2013) Hydrothermal synthesis of hydroxyapatite nanoparticles and their protein adsorption behavior. Journal of the Ceramic Society of Japan 121(1417): 797-801.
- Sinitsyna OV, Veresov AG, Kovaleva ES, Kolenk, YV, Putlyaev VI, et al. (2005) Synthesis of hydroxyapatite by hydrolysis of α-Ca3(PO4)2. Russian Chemical Bulletin 54: 79-86.
- Durucan C, Brown PW (2000) α-Tricalcium phosphate hydrolysis to hydroxyapatite at and near physiological temperature. Journal of Materials Science: Material in Medicine 11(6): 365-371.
- Wang MC, Chen HT, Shih WJ, Chang HF, Hon MS, et al. (2015) Crystalline size, microstructure and biocompatibility of hydroxyapatite nanopowders by hydrolysis of calcium hydrogen phosphate dehydrate (DCPD). Ceramics International 41(2): 2999-3008.
© 2023 Luddin N. This is an open access article distributed under the terms of the Creative Commons Attribution License , which permits unrestricted use, distribution, and build upon your work non-commercially.
 a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in
a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in 







.jpg)






























 Editorial Board Registrations
Editorial Board Registrations Submit your Article
Submit your Article Refer a Friend
Refer a Friend Advertise With Us
Advertise With Us
.jpg)






.jpg)














.bmp)
.jpg)
.png)
.jpg)










.jpg)






.png)

.png)



.png)






