- Submissions

Full Text
Modern Research in Dentistry
Investigating the Effect of the Time Elapsed after Treatment on the Severity of Dry Mouth in Patients Experiencing Dry Mouth due to Head and Neck Cancer Treatment
Veysel Eratilla1* and İhsan Kaplan2
1Faculty of Dentistry, Prosthetic Dentistry Department, Turkey
2Department of Nuclear Medicine, Turkey
*Corresponding author: Veysel Eratilla, Faculty of Dentistry, Prosthetic Dentistry Department, Batman University, Turkey
Submission: June 23, 2023;Published: July 26, 2023

ISSN:2637-7764Volume7 Issue5
Introduction
Saliva is a liquid produced in the salivary glands located in the mouth. It is an aqueous substance composed of water, electrolytes, enzymes, and other proteins. Saliva helps moisten the mouth and lubricate food for easier swallowing. It also contains enzymes that start the digestive process, breaking down food in the mouth. In addition, saliva helps protect teeth and gums by removing bacteria and food particles that can cause tooth decay and gum disease. Saliva also plays a role in the sense of taste, as it carries taste molecules to the taste buds on the tongue. In general, saliva plays an important role in maintaining oral health and aiding in digestion [1,2].
Saliva plays a very important role in protecting teeth from decay. It helps neutralize the acids produced by bacteria in the mouth and washes away food particles that can stick to the teeth and cause decay. Saliva also contains minerals such as calcium and phosphate, which can help repair acid-damaged tooth enamel. Also known as xerostomia, dry mouth can be caused by a variety of factors, including medications, certain medical conditions, and radiation therapy. When there is not enough saliva in the mouth, harmful bacteria can grow and produce more acid, causing tooth decay [3]. In addition, the composition of saliva can affect the risk of tooth decay. Saliva with low pH or acidity can encourage the growth of harmful bacteria and increase the risk of tooth decay. Certain medications or medical conditions can increase the risk of tooth decay by changing the pH of saliva. In summary, saliva plays an important role in protecting teeth from decay, and reductions in saliva production or changes in saliva composition can increase the risk of tooth decay [4].
Dry mouth, also known as xerostomia, is a condition in which the salivary glands in the mouth do not produce enough saliva. Xerostomia symptoms may include dryness or stickiness in the mouth, persistent thirst, difficulty swallowing, dry or sore throat, bad breath, and changes in taste and smell. Xerostomia can increase the risk of developing tooth decay, gum disease, and oral infections. It can also make it difficult to speak, eat, or wear dental appliances such as dentures [5,6]. Dry mouth has many causes, including medications, radiation therapy, aging, and nerve damage. Among these reasons, radiotherapy stands out as the most common and long-lasting factor. Information on how dry mouth starts after radiation therapy, how long it lasts, and which age group it affects more are important in terms of making our treatment plan in advance and ensuring that the patient is least affected [7,8]. The effect of dry mouth in patients treated with radioactive iodine was the focus of our study. Specifically, we investigated how the time elapsed after patients received RAI treatment which affected dry mouth in these individuals.
Material and Method
This study was retrospectively planned, and ethical approval was obtained by the clinical research ethics committee consisting of University Faculty Members (Date: 16.02.2022, Decision No: 2022-02). All procedures are carried out in accordance with the ethical rules and principles of the Declaration of Helsinki.
Our study, which included thyroid cancer patients, was selected from among the patients who applied to our Thyroid Polyclinic in the Nuclear Medicine Department of the Training and Research Hospital between January 2021 and December 2022. The salivary gland scintigraphies performed on those who developed dry mouth during the control period after discharge from those who were admitted to our clinic for thyroid cancer and received highdose Radioactive Iodine (RAI) 131 treatment were retrospectively analyzed. During this study, patients who had received RAI treatment (>30mCI RAI), were hospitalized in our clinic, and had salivary gland scintigraphy were included in the study. Those who had a disease and had communication problems were excluded from the study.
The necessary apparatus for preparing and drinking 5 ml of lemon juice was prepared to be used in the pre-scintography shooting, and information about the shooting phase was given to the patient. During the shooting, the patient was advised not to speak or move, and the requirements were met. Imaging in the supine position was required by each patient imaging. The SPECT device was used by adjusting the window width to 20% and peak 140kev. For the imaging of the salivary glands, 10mCi (370MBq) 99mTc pertechnetate was administered intravenously through the cubital vein, and the patient was given a lemon juice drink, which we prepared before, at the 15th minute of the 30-minute imaging. Evaluation of the images was done by 2 nuclear medicine specialists. The Regions of Interests (ROI) of the patients were drawn in such a way that it penetrated the bilateral parotid and submandibular glands, and measurements were made semi-quantitatively. The functions of the salivary glands were graded according to the filling and discharge curves after the measurements and classified into 3 groups according to their severity.
Result
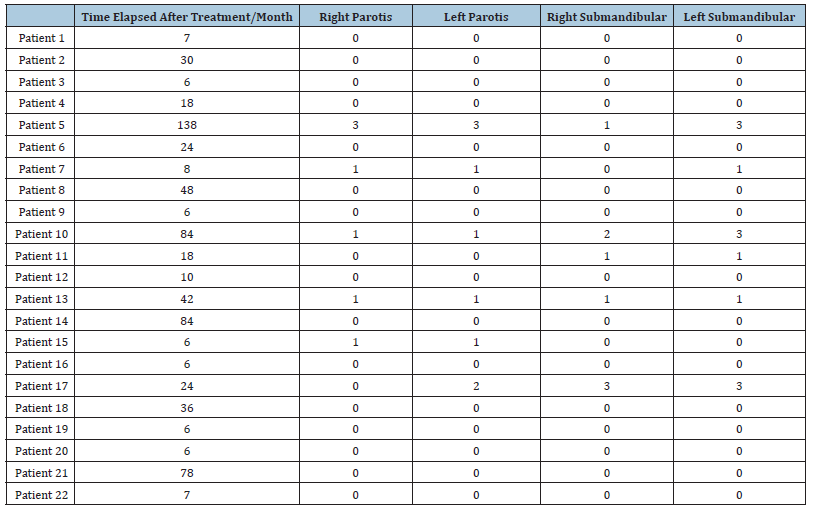
(Table 1)
Table 1: The following dial shows the degree of deterioration:
0= normal
1= slight distortion
2= Moderate Distortion
3= Severe Deterioration
Statistical Analysis
(Figure 1) Statistical analysis of our research data was performed using the IBM SPSS 21.0 for Windows statistical program. The mean ± Standard Deviation (SD) values of continuous variables and the number and percentage (%) of categorical variables were presented. The χ2 (Chi-square) test was used to evaluate the differences between categorical variables. The Spearman correlation test was used to determine the relationships between the variables. The p<0.05 value was considered statistically significant for the two-way hypotheses (Table 2).
Figure 1:
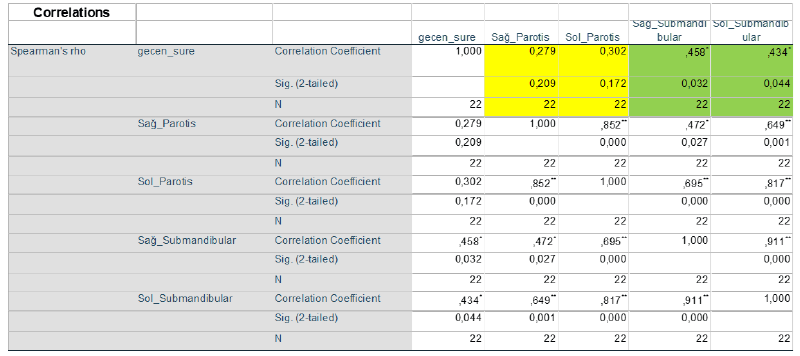
Table 2:
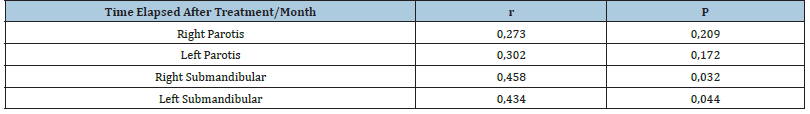
No correlation was found between the right and left Parotid Glands and the time elapsed. Therefore, we concluded that Time Change had no effect on the right and left Parotid Glands. However, a positive correlation was found between the time and the right and left lower mandibular glands. This shows that the changes in the Right and Left Mandibular Glands increase with the passage of time (Figure 2).
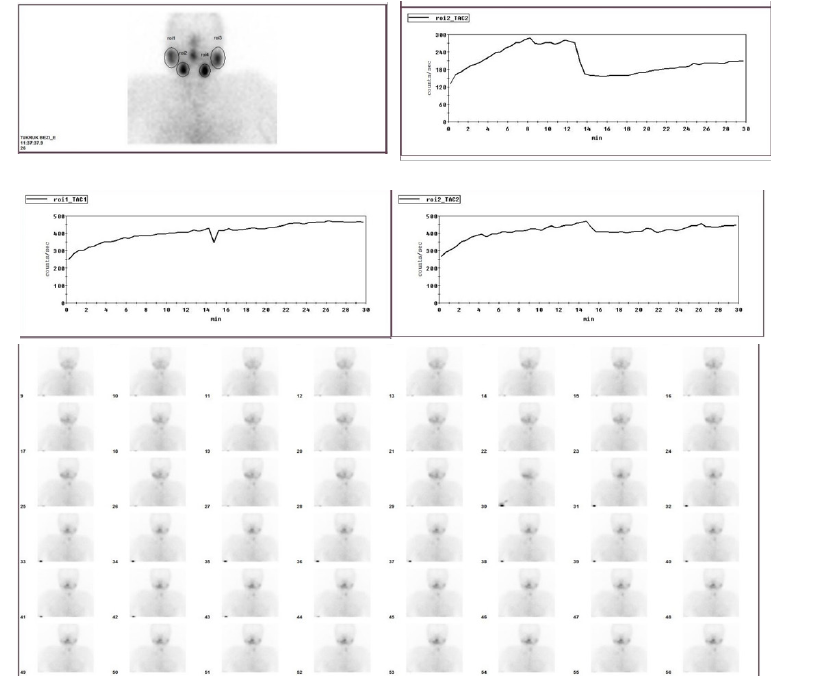
Figure 2:A 52-year-old female patient was treated 84 weeks ago. Salivary gland scintigraphy taken from a patient with dry mouth.
Conclusion
In our study, the occurrence of dry mouth after radioactive iodine in patients is supported by many previous articles [9]. It was observed that many factors were evaluated together in these studies, and the lack of specific studies in the literature is striking. In our study, one of the factors, the time elapsed after treatment, was examined and will be the basis for future studies. In our study, it was observed that patients had difficulties during many functions such as eating, talking or tasting.
Oda B et al. [10] reported findings in a large-scale study investigating dry mouth in long-term survivors of head and neck cancer patients treated with radiation therapy. They stated that all of the patients complained of dry mouth as a result of radiation therapy, and 28% of them were very severe [10]. About thirty percent of the mucin content of saliva is produced by the sublingual and submandibular glands. Mucin is the most important component of saliva to maintain the lubricity of the mucosa. Lubrication of oral mucosal membranes is essential for oral comfort and prevention of xerostomia [11]. Since the submandibular glands were affected which is important for patient comfort. The importance as to how long xerostomia will last and the effects of change over time.
Cheng VS et al. [11] conducted a study on 18 patients with head and neck cancer who received radiation therapy. Stimulated saliva samples were collected from the parotid gland before radiation and 1 and 8 months after radiation. When 100% of the parotid gland was irradiated, no saliva was produced 1 month after radiation. They reported that dry mouth persisted and did not decrease during this time. When retested at 4-8 months it remained the same. In our study, we found that the dry mouth did not go away over elapsed time [12].
In their study, Lars Franzén et al. [13] explained that radiotherapy of tumors in the head and neck region usually involves the salivary glands in the treatment volume, which resulted in dryness and discomfort. As a result of the study, all but one of the patients who received low doses reported that there was an improvement in secretion that started after 2 months with a continuous improvement in salivary flow for up to 18 months. In our study, we found that the patients who received high doses did not have dry mouth despite the elapsed time [13].
In their study on the long-term effects of radiotherapy on taste and salivary function in humans, Mossman K et al. [14] examined 13 patients who had undergone radiation therapy for head and neck tumors 1-7 years ago and reported measurable dysfunction of the salivary glands in every patient who received radiotherapy. We have also supported that the functional deficiency persisted, especially at high doses, despite the passage of 7 years [14].
Chia-Yung Lin et al. [15] in their multidimensional study on the effects of radiotherapy on salivary gland function in patients with head and neck cancer, looked at the DMF-T index, which is calculated based on the surfaces of the teeth and expressed as the number of caries per person and the number of surfaces affected by its results, and after 5 years, the DMF-T value of the control group was examined and the found within these patients who received radiotherapy did not change, but the values in the patients who received radiotherapy changed. Thus, they also revealed the different results of dry mouth in the postoperative period. Salivary flow rate was analyzed in a mixed model in the repeat measurement group. The salivary flow rate before radiotherapy was 7.18. It decreased to 2.71 at 1 month after radiotherapy and to a minimum of 1.5 at 2 months after radiotherapy. They reported that it was around 3.5 after 10-12 months following radiotherapy and probably would not return to its old flow even after 5 years. In our study, we found that there was no complete recovery after treatment [15].
According to Blanco et al. [16] the salivary flow rate returned to a certain amount from 2.15 to 3.15 (6-12 months after radiotherapy). In addition, Möller et al. [17] reported that the flow rate would gradually improve 4 months after radiotherapy but would not return to its original level. Both studies reported that any improvement was limited, and dry mouth persisted. In the results of the measurement groups, they stated that age, gender and the time interval after radiotherapy were not significant predictive factors for salivary flow rate. This supports the results of our study.
Further, Epstein et al. [18] reported that hyposalivation develops due to progressive loss of salivary gland function in approximately 70% of patients receiving head and neck radiotherapy, which can be observed in the first weeks of treatment and often persists throughout the patient’s life [18]. Different levels of hyposalivation in patients after radiotherapy have been observed. They stated that there is a direct relationship between the decrease in glandular function and the increased radiation dose, but they reported that the function is reversible at low doses. In our study, we found that the function did not return after exposure to high doses [19-21]. Studies have reported a macroscopically detectable loss of salivary gland structure as a result of radiotherapy. This decrease was proportional to the radiation dose. In one study, the weights of the parotid and submandibular glands decreased to 60% and 40% of the initial value, respectively [22]. Further, they confirmed the presence of acinar atrophy, and as a result, dry mouth continued [23].
Saliva, which is vital for oral and dental health as well as for digestion and nutrition, plays a very important role in maintaining general health. Healthy salivary glands and proper saliva secretion are key factors in promoting good oral health and promoting proper nutrition. Our study shows that knowing the salivary gland function status, which is of vital importance in terms of comfort in patients undergoing radiotherapy, cannot be ignored. Knowing the structural changes and functional status observed in the salivary glands will guide the proof of the short and long-term effectiveness of the investigated treatments. In our study, it was found that highdose RAI treatment caused salivary gland dysfunction for long periods and this was irreversible. With collaboration between radiotherapists and dentists, it is clear that these patients need careful dose planning and close follow-up. In these multidisciplinary treatment plans, the advice of relevant field experts should be taken.
References
- Eratilla V (2001) Investigation of parotid gland function changes caused by dry mouth in patients receiving high-dose radioactive iodine treatment. International Journal of Basic and Clinical Studies 10(2): 66-74.
- Eratilla V, Doğan MS, Eratilla E (2018) Seroprevalence of hepatitis and HIV in people admitted to Diyarbakir state hospital. Journal of Harran University Medical Faculty 15(3): 241-244.
- Stookey GK (2008) The effect of saliva on dental caries. The Journal of the American Dental Association 139: 11S-17S.
- Lenander-Lumikari M, Loimaranta V (2000) Saliva and dental caries. Advances in Dental Research 14(1): 40-47.
- Cooper JS, Fu K, Marks J, Silverman S (1995) Late effects of radiation therapy in the head and neck region. Int J Radiat Oncol Biol Phys 31(5): 1141-1164.
- Beeken L, Calman F (1994) A return to “normal eating” after curative treatment for oral cancer. What are the long-term prospects. Oral Oncol Eur J Cancer 30B (6): 387-392.
- Shannon IL, Trodahl JH, Starcke EN (1978) Radiosensitivity of the human parotid gland. Proc Soc Exp Biol Med 157(1): 50-53.
- Zhao L, Xu J, Li S, Li B, Jia M, et al. (2022) Resveratrol alleviates salivary Bez dysfunction induced by ovariectomy in rats. Biochemical and Biophysical Research Communications 630: 112-117.
- Liu RP, Fleming TJ, Toth BB, Keene HJ (1990) Salivary flow rates in patients with head and neck cancer 0.5 to 25 years after radiotherapy. Oral Surg Oral Med Oral Pathol 70(6): 724-729.
- Wijers OB, Levendag PC, Braaksma MM, Boonzaaijer M, Visch LL, et al. (2002) Patients with head and neck cancer cured by radiation therapy: A survey of the dry mouth syndrome in long‐term survivors. Head & Neck 24(8): 737-747.
- Simcock R, Fallowfield L, Monson K, Solis-Trapala I, Parlour L, et al. (2013) ARIX: a randomised trial of acupuncture v oral care sessions in patients with chronic xerostomia following treatment of head and neck cancer. Annals of Oncology 24(3): 776-783.
- Cheng VS, Downs J, Herbert D, Aramany M (1981) The function of the Parotid gland following radiation therapy for head and neck cancer. International Journal of Radiation Oncology Biology Physics 7(2): 253-258.
- Franzén L, Funegård U, Ericson T, Henriksson R (1992) Parotid gland function during and following radiotherapy of malignancies in the head and neck: A consecutive study of salivary flow and patient discomfort. European Journal of Cancer 28(2-3): 457-462.
- Mossman K, Shatzman A, Chencharick J (1982) Long-term effects of radiotherapy on taste and salivary function in man. International Journal of Radiation Oncology Biology Physics 8(6): 991-997.
- Lin CY, Ju SS, Chia JS, Chang CH, Chang CW, et al. (2015) Effects of radiotherapy on salivary gland function in patients with head and neck cancers. Journal of Dental Sciences 10(3): 253-262.
- Blanco AI, Chao KC, El Naqa I, Franklin GE, Zakarian K, et al. (2005) Dose-volume modeling of salivary function in patients with head-and-neck cancer receiving radiotherapy. International Journal of Radiation Oncology Biology Physics 62(4): 1055-1069.
- Möller P, Perrier M, Ozsahin M, Monnier P (2004) A prospective study of salivary gland function in patients undergoing radiotherapy for squamous cell carcinoma of the oropharynx. Oral Surgery, Oral Medicine, Oral Pathology, Oral Radiology, and Endodontology 97(2): 173-189.
- Epstein JB, Thariat J, Bensadoun RJ, Barasch A, Murphy BA, et al. (2012) Oral complications of cancer and cancer therapy: from cancer treatment to survivorship. CA Cancer J Clin 62(6): 400-422.
- Deasy JO, Moiseenko V, Marks L, Chao KC, Nam J, et al. (2010) Radiotherapy dose-volume effects on salivary gland function. International Journal of Radiation Oncology Biology Physics 76(3): S58-S63.
- Li Y, Taylor JM, Ten Haken RK, Eisbruch A (2007) The impact of dose on parotid salivary recovery in head and neck cancer patients treated with radiation therapy. International Journal of Radiation Oncology Biology Physics 67(3): 660-669.
- Murdoch-Kinch CA, Kim HM, Vineberg KA, Ship JA, Eisbruch A (2008) Dose-effect relationships for the submandibular salivary glands and implications for their sparing by intensity modulated radiotherapy. International Journal of Radiation Oncology Biology Physics 72(2): 373-382.
- Nagler RM, Baum BJ, Miller G, Fox PC (1998) Long-term salivary effects of single-dose head and neck irradiation in the rat. Archives of Oral Biology 43(4): 297-303.
- Radfar L, Sirois DA (2003) Structural and functional injury in minipig salivary glands following fractionated exposure to 70 Gy of ionizing radiation: an animal model for human radiation-induced salivary gland injury. Oral Surgery, Oral Medicine, Oral Pathology, Oral Radiology, and Endodontology 96(3): 267-274.
© 2023 Veysel Eratilla. This is an open access article distributed under the terms of the Creative Commons Attribution License , which permits unrestricted use, distribution, and build upon your work non-commercially.
 a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in
a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in 







.jpg)






























 Editorial Board Registrations
Editorial Board Registrations Submit your Article
Submit your Article Refer a Friend
Refer a Friend Advertise With Us
Advertise With Us
.jpg)






.jpg)














.bmp)
.jpg)
.png)
.jpg)










.jpg)






.png)

.png)



.png)






