- Submissions

Full Text
Modern Research in Dentistry
Evaluating The Effects of Probiotics After SRP on Generalized Moderate to Severe Chronic Periodontitis - A Randomized Clinical Trial
Naser Sargolzaie1, Negin Riahi2, Negar Bahrami Taghanaki2, Kimia Kelidari2* and Milad Bonyatpour2
1Department of Periodontics, Iran
1School of Dentistry, Iran
*Corresponding author: Kimia Kelidari, School of Dentistry, Mashhad, Iran
Submission: April 05, 2022;Published: July 29, 2022

ISSN:2637-7764Volume7 Issue2
Abstract
Aim: Periodontitis is the inflammation of teeth surrounding tissues caused irreversibly by infection. Oral hygiene education, Scaling and Root Planning (SRP), antibiotics and surgical procedures are the existing treatment methods. However, they are not long-lasting. Therefore, we aimed to evaluate the effect of probiotic administration as mouthwash after SRP on periodontal condition in patients with generalized moderate to severe chronic periodontitis. Materials and methods: In this study which was a double-blinded randomized clinical trial, 51 eligible patients with generalized moderate to severe chronic periodontitis who were referred to Mashhad Faculty of dentistry, were randomly allocated to test (SRP+ probiotics (FamiLact, n=26)) or control (SRP+placebo (n=25)) group. All the subjects were instructed to use the capsules as mouthwash once a day for 20 days. At the initial phase of the study, we used Clinical Attachment Loss (CAL) for determination of generalized moderate to severe chronic periodontitis. Afterwards, we employed Bleeding on Probing (BOP) and Pocket Probing Depth (PPD) at baseline and after 20-day course of treatment. Results: After the baseline treatments and 20-day period of administration of capsules, the test group demonstrated a 34.7% reduction in BOP index and a 28.76% reduction was observed in the control group (P-value=0.001, difference=6%). The test group also showed a 1.68mm reduction in PPD index which was significantly higher than that of the control group with 0.88mm reduction (P-value=0.0001, difference=0.79mm). Conclusion: According to the results of our study and significant difference of parameters improvement between the test and control groups, it is concluded that topical administration of probiotics after SRP can help decrease periodontal inflammation in the short-term..
Keywords: Chronic periodontitis; Gingiva; Probiotic; Scaling and root planning; Oral health; Mouthwash
Abbreviations:SRP: Scaling and Root Planning; CAL: Clinical Attachment Loss; BOP: Bleeding on Probing; PPD: Pocket Probing Depth; GI: Gingival Index; PI: Plaque Index; BI: Bleeding Index; MGI: Modified Gingival Index; CAG: Clinical Attachment Gain; GBI: Gingival Bleeding Index
Introduction
Periodontitis is an irreversible infection-driven inflammatory and multifactorial disease that encompasses hard and soft teeth supporting tissues, inflammatory and immune responses [1,2]. The chronic periodontitis is categorized into three groups based on the attachment loss of gingiva: mild (1-2mm), moderate (3-4mm), severe (5mm and more) [3]. The reference method for treatment is mechanical debridement by Scaling and Root Planning (SRP) [4]. In addition, multiple supportive medications for SRP exist such as antibiotics, essential oils, probiotics, laser, photodynamic therapy and in advanced cases surgery [1,4]. The chosen adjunctive therapy in this research is to employ probiotics. Probiotics is the name of the bacteria with beneficial effects for humans and animals [5]. Probiotics are Live micro-organisms which have a beneficial health effect on the host when used in adequate amounts(WHO/FAO) [6]. These bacteria can be very useful for treating periodontal diseases because of their anti-microbial and anti-inflammatory effects [7]. The mechanism of action vary according to the specific strain or combination of strains used [2].
Several studies confirmed the effectiveness of probiotics in treating periodontitis, but only a few studies evaluated the effect of probiotics in treating chronic periodontitis. Some of these studies were randomized controlled trials in which significant reduction in clinical periodontal parameters was gained with the use of probiotics and their results recommend the use of probiotics as an additional treatment to SRP or a substitute treatment for SRP [7-10]. Furthermore, recent meta-analyses and systematic reviews showed that probiotics are effective in treating chronic periodontitis patients [11-13]. Among these, Vives-soler et al. [12] conducted a systematic review including nine articles. They concluded that probiotics can be helpful together with manual debridement in patients with chronic periodontitis but further studies considering the dose, administration method and probiotic strains are needed. Given that none of these studies have been conducted in Iran, particularly northeast of Iran and multiple factors including genetics, environment and behavioral factors are associated with periodontitis [1], the necessity of conducting such a study on the northeast population of Iran is of high importance. The aim of the present study, therefore, was to evaluate the effectiveness of probiotic mouthwash (FamiLact) after SRP on periodontal condition of generalized moderate to severe chronic periodontitis patients in the northeast of Iran.
Materials and Methods
This double-blinded placebo-controlled randomized clinical trial was conducted on patients who were referred to the periodontology department at Mashhad faculty of dentistry and some other dental clinics at Mashhad, between 2017 and 2018. Ethical approval was acquired from Mashhad University of medical sciences’ medical ethics committee and registered at IR.MUMS. DENTISTRY.REC.1397.029. Patient population needed for the study was determined based on the Teughels et al. [7] study. Participants were randomly divided into two interventional groups each consisting 30 members using block randomization method. One group received SRP+ probiotic capsules (FamiLact made by Tehran Zisttakhmir company containing seven different bacterial species) and the other group received SRP+ placebo capsules (containing starch).The inclusion criteria were as follows: age between 35 and 50, generalized moderate to severe chronic periodontitis; Clinical Attachment Loss (CAL)>3mm [14] or radiographic clues confirming this periodontal situation (bone loss more than 20% of the attachment surface [15] when at least 30% of the dentition is affected [3] and Pocket Probing Depth (PPD)>4mm. Smokers, patients with systemic infection, diabetes, autoimmune diseases and immune deficiency disorders, pregnant and lactating women, patients with the history of periodontal treatment, antibiotic therapy, administration of NSAIDS and corticosteroid medications within the last 3 months [16] were excluded from the study. Eventually, 51 eligible individuals were enrolled in this study. It should be noted that 26 patients including 12 males and 14 females participated in the test group and 25 patients including 12 males and 13 females in the control group. Initially, we used Clinical Attachment Loss (CAL) for determining generalized moderate to severe chronic periodontitis. Age and gender parameters were also employed for balancing the groups. Bleeding On Probing (BOP) [17] and Pocket Probing Depth (PPD) were recorded at baseline as well as 20 days after therapy.
After written and verbal explanation of the protocol of the study to all participants who signed an informed consent form, we recorded each patient’s information, their code and the studied indicators i.e., BOP and PPD in the individual prepared tables. In the next step, etiotropic phase involving scaling and root planning was done besides oral hygiene education by the same calibrated examiner at the same time. It should be noted that SRP was performed thoroughly and similarly for all subjects and modified bass technique was used as their oral hygiene instruction. Finally, every participant was given a coded pack containing 20 capsules (probiotic or placebo) and they were instructed to dissolve one capsule in two tablespoons of tepid water and use the solution as mouthwash once a day for a minute after brushing and flossing their teeth. Three weeks after the beginning of the treatment period, subjects were invited and aforesaid indicators were remeasured the same way by the former calibrated examiner who was blinded to the content of capsules. After the study completion, based on the code of each participant, the designation of the different groups was revealed (test/control). To demonstrate central tendency and index of dispersion, data were described using appropriate tables and graphs. Data analysis was done by independent samples T test, paired samples T test or their non-parametric equivalents. To control confounding variables, we used analysis of covariance. Level of statistical significance was considered 5%. PPD was the outcome which was employed to calculate participants’ population due to its importance, rate of change and being the best criterion for healing progress.
Results
Age and sex distribution of patients
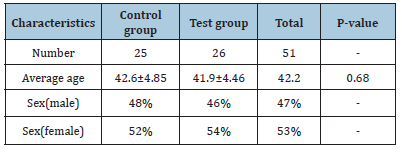
In this study, 25 patients with generalized moderate to severe chronic periodontitis participated as the control group and 26 Patients with the same periodontal condition participated as the test group. According to (Table 1), distribution age of people was homogenous, and no significant difference was found between them (P-value=0.68).
Table 1: Background characteristics of the subjects in the control and test groups at the beginning of the study.
Statistical findings related to clinical parameters
According to (Table 2) in the test group, after 21 days of probiotic use, the mean BOP decreased by half (49.3%) which Demonstrates the significant effect of probiotics on the treatment of generalized moderate to severe chronic periodontitis (P-value <0.001). In the control group in which participants used placebo capsules, after the end of the treatment period, the mean BOP decreased by a little less than half (42.1%)(P-value<0.001). This table also shows the mean PPD before and after the probiotic treatment period in the case and control groups. In the case group, after using probiotics, the mean PPD decreased significantly (28.7%) (P-value<0.001). The control group who received Placebo capsule had a slight PPD reduction after the treatment period compared to the case group (15.55%) (P-value<0.001). (Table 3) Compares the reduction of the two clinical parameters of BOP and PPD in the two experimental groups. The amount of reduction of both parameters is higher in the test group. Although the mean differences in BOP and PPD reduction (P-value=0.001, 0.0001) between the two groups were noteworthy, it was slightly more significant for PPD. 34.7% reduction in bleeding on probing and 1.68mm reduction in the pocket probing depth of people who used probiotics in addition to SRP compared to a decrease of 28.76% in bleeding on probing and 0.88mm decrease in pocket probing depth of individuals receiving SRP alone indicates that probiotics are effective in treating chronic periodontitis.
Table 2: Changes in parameters related to BOP and PPD index in the control and test groups.
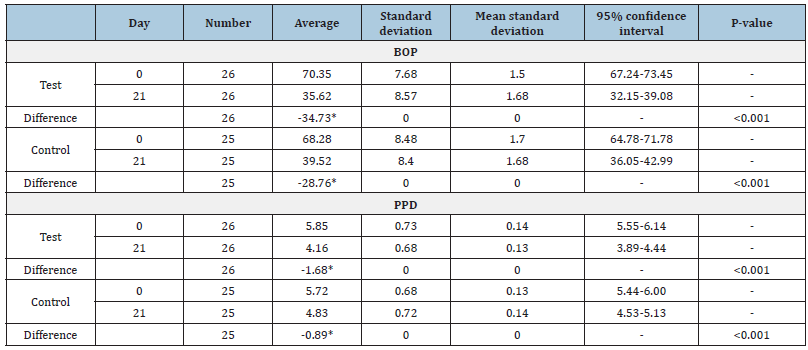
Table 3: Comparison of changes in clinical parameters of the control and test groups at the end of the study.

Discussion
This double-blinded placebo-controlled randomized clinical trial aimed to evaluate the effect of topical use of probiotics after SRP on periodontal condition of patients with generalized moderate to severe chronic periodontitis. The results demonstrated that topical administration of probiotics diminishes periodontitis by means of changing the subgingival microflora at least for a short duration. At the end of this study, BOP and PPD were reduced in both the test and control groups and the reduction of the two aforesaid indexes was higher in the test group but it is important to note that the mean difference in PPD reduction between the two groups was more significant than the mean difference in BOP reduction. This might be because of the beneficial effect of SRP and the removal of etiological factors of plaque accumulation and pathogenic microorganisms from periodontal attachments. The positive effect of probiotics is proved due to the inflammation decrease between the test and the control group, though. To the best of our knowledge, this is the first trial evaluating the effect of probiotic administration as mouthwash in patients with chronic periodontitis in Iran, especially the northeast of Iran. Additionally, no other study out of the studies discussed in the present article used probiotic FamiLact containing 7 bacterial species of Lactobacillus, Bifidobacterium and Streptococcus genus. A study with results different from our study is [6] one-year placebo-controlled RCT which can be compared to our study due to evaluating the effects of probiotics on BOP and PPD and measuring the two indexes on day 21. 40 patients including 18 males and 22 females, who had at least two teeth with a PPD of 5-7mm, participated in their study. They found that after using probiotic lozenges, on days 21, 90, 180 and 360, all parameters including Gingival Index (GI), Plaque Index (PI), BOP and PPD were significantly lower in the test group in comparison with the control group. Comparing the two studies, at the same period (21 days), BOP showed lower reduction in both groups of our study (test -34.7%, control -28.7%, P-value=0.001) in comparison with Tekce study (test -67.4%, control -63%, P-value=0.001). One of the probable reasons for this difference might be the fact that our participants were different from Tekce’s in terms of genetics, environmental and behavioral factors. In contrast, at the same period, PPD showed higher reduction in both groups of our study (test -1.68mm, control -0.88mm, P-value=0.0001) compared with their study (test -1.20mm, control -0.76mm, P-value=0.001). In addition, mean difference in PPD reduction between the two groups was significantly higher in our study (0.79mm) in comparison with their study (0.44mm) which could be because of the different type of probiotic used. We utilized FamiLact in our study which contains more various bacterial species than BioGia Prodentis used by Tekce that consists of Lactobacillus reuteri strains. It is also important to note that the form of probiotic was different in our study (mouthwashes) compared with their study (lozenges) [9].
Other clinical parameters than those we measured in our study that can represent the effect of probiotic on periodontal condition are PI, Bleeding Index (BI), Modified Gingival Index (MGI) and clinical Attachment Gain (CAG) Which were measured in Penala et al. [10] study. They performed a trial with a smaller sample size (32 chronic periodontitis patients) and a shorter probiotic mouthwash usage period (15 days). After performing SRP for all patients, the same as our study, the test and the control group received probiotic and placebo mouthwashes respectively. They observed that PI, BI and MGI showed significant improvement at 3 months followup appointment when comparing the test and control groups but improvement in PPD (aside from PPD in moderate pockets of the test group) and CAG was insignificant. The reasons might be shorter probiotic usage period compared with similar studies and performing follow-up a long time after the start of the study (3 months). Martin-Cabezas et al. [11] analyzed the effect of probiotics on factors such as Gingival Bleeding Index (GBI) in their meta-analysis, in addition to parameters measured in our study. This meta-analysis was performed on three selected articles and the results showed that the effects of probiotics on periodontal parameters was significant only in moderate to deep pockets and among the clinical parameters, bleeding related ones including BOP and GBI showed the most significant changes. Mean difference in BOP between the two groups in Martin meta-analysis showed a significantly higher reduction (-14.66%) than that of our study (-6%). A reason might be the difference in the type, form and daily intake of the probiotic used. They used 2 L. reuteri lozenges per day but we used one FamiLact capsule as mouthwash per day. Moreover, the effect of genetics and environment cannot be disregarded.
A recent study in which the effect of probiotic as a complement to SRP in patients with chronic periodontitis was reviewed, belongs to Vives-soler et al. [12] They concluded that probiotics cannot improve overall PPD. Pockets with a depth of 4 to 6 mm showed higher reductions in the test groups and the need for surgery had been decreased, though. Our study was also performed on patients with PPD of more than 4mm and proved the positive effect of probiotics on the decrease in PPD in generalized moderate to severe chronic periodontitis patients. Also, Song et al. [13] reviewed the three selected articles of Martin-Cabezas et al. [11] study in part of their meta-analysis in order to evaluate PPD reduction after a 21- day period of using probiotics. Their study can be compared to our study because of the similarity in the evaluated clinical parameter and the period. According to the results of their meta-analysis, mean difference in PPD reduction between the test and the control group in their study (0.61mm) was a bit lower than that of our study (0.79mm). A possible reason could be the difference in the type of probiotic used in the three studies compared with our study but in general, the difference in PPD reduction between the test and the control group was significant in both studies and represented the considerable effect of probiotic on periodontium [13]. A very few studies are in conflict with our study and all the other aforesaid studies in terms of results such as Morales et al. [18] study. Their participants were divided into three treatment groups including probiotic, antibiotic and placebo, and the results demonstrated that either probiotic or antibiotic exhibits a similar outcome as SRP alone in treating chronic periodontitis.
Regarding the beneficial effects of topical use of probiotics in reducing periodontal inflammation, it is recommended that patients with gastrointestinal diseases, who take probiotic pills, use them as a mouthwash before swallowing them. Therefore, they benefit from probiotics effect on their oral health in addition to their gastrointestinal health. The strengths of this study were the homogeneity of patients in terms of number, age, gender and clinical parameters and adequate sampling size due to the large number of patients with periodontitis. The major limitation of the present study was the short study period. Another limitation was that most of the probiotic species in our capsules originated from gut. According to recent studies [19], probiotics containing indigenous oral microorganisms are more probable to be effective. We recommend that Future studies consider probiotics efficacy in terms of dose and consumption period, administration method, microbial species and strains, and specialized effect on certain periodontal bacteria.
Conclusion
Regarding the considerable difference in the treatment outcomes between the probiotic group and the placebo group, it is concluded that topical application of probiotics after SRP can decrease periodontal inflammation by altering the subgingival microflora in generalized moderate to severe chronic periodontitis patients at least in the short-term.
Acknowledgement
This study was supported by Research Deputy of Mashhad University of Medical Sciences. We would like to thank our patients for their voluntary participation.
References
- Könönen E, Gursoy M, Gursoy UK (2019) Periodontitis: A multifaceted disease of tooth-supporting tissues. Journal of Clinical Medicine 8(8): 1135.
- Gupta G (2011) Probiotics and periodontal health. J Med Life 4(4): 387-394.
- Newman MG, Takei HH, Klokkevold PR, Carranza FA (2019) Newman and Carranza’s Clinical Periodontology, Elsevier, Amsterdam, Netherlands, p. 65.
- Hu D, Zhong T, Dai Q (2021) Clinical efficacy of probiotics as an adjunctive therapy to scaling and root planning in the management of periodontitis: A systematic review and meta-analysis of randomized controlled trails. J Evid Based Dent Pract 21(2): 101547.
- Hotel ACP, Cordoba A (2001) Health and nutritional properties of probiotics in food including powder milk with live lactic acid bacteria. Prevention 5(1): 1-10.
- Teughels W, Loozen G, Quirynen M (2011) Do probiotics offer opportunities to manipulate the periodontal oral microbiota? J Clin Periodontol 38(11): 159-77.
- Teughels W, Durukan A, Ozcelik O, Pauwels M, Quirynen M, et al. (2013) Clinical and microbiological effects of Lactobacillus reuteri probiotics in the treatment of chronic periodontitis: A randomized placebo-controlled study. J Clin Periodontol 40(11): 1025-1035.
- Vivekananda MR, Vandana KL, Bhat KG (2010) Effect of the probiotic Lactobacilli reuteri (Prodentis) in the management of periodontal disease: A preliminary randomized clinical trial. J Oral Microbiol 2: 2.
- Tekce M, Ince G, Gursoy H, Dirikan Ipci S, Cakar G, et al. (2015) Clinical and microbiological effects of probiotic lozenges in the treatment of chronic periodontitis: A 1-year follow-up study. J Clin Periodontol 42(4): 363-372.
- Penala S, Kalakonda B, Pathakota KR, Jayakumar A, Koppolu P, et al. (2016) Efficacy of local use of probiotics as an adjunct to scaling and root planing in chronic periodontitis and halitosis: A randomized controlled trial. J Res Pharm Pract 5(2): 86-93.
- Martin Cabezas R, Davideau JL, Tenenbaum H, Huck O (2016) Clinical efficacy of probiotics as an adjunctive therapy to non-surgical periodontal treatment of chronic periodontitis: A systematic review and meta-analysis. J Clin Periodontol 43(6): 520-530.
- Vives-Soler A, Chimenos-Küstner E (2020) Effect of probiotics as a complement to non-surgical periodontal therapy in chronic periodontitis: A systematic review. Med Oral Patol Oral Cir Bucal 25(2): 161-167.
- Song D, Liu X-R (2020) Role of probiotics containing Lactobacillus reuteri in adjunct to scaling and root planing for management of patients with chronic periodontitis: A meta-analysis. Eur Rev Med Pharmacol Sci 24(8): 4495-4505.
- Socransky SS, Haffajee AD, Goodson JM, Lindhe J (1984) New concepts of destructive periodontal disease. J Clin Periodontol 11(1): 21-32.
- López NJ, Socransky SS, Da Silva I, Japlit MR, Haffajee AD (2006) Effects of metronidazole plus amoxicillin as the only therapy on the microbiological and clinical parameters of untreated chronic periodontitis. J Clin Periodontol 33(9): 648-660.
- López NJ, Gamonal JA, Martinez B (2000) Repeated metronidazole and amoxicillin treatment of periodontitis. A follow-up study. J Periodontol 71(1): 79-89.
- Meitner SW, Zander HA, Iker HP, Polson AM (1979) Identification of inflamed gingival surfaces. J Clin Periodontol 6(2): 93-97.
- Morales A, Gandolfo A, Bravo J, Carvajal P, Silva N, et al. (2018) Microbiological and clinical effects of probiotics and antibiotics on nonsurgical treatment of chronic periodontitis: A randomized placebo-controlled trial with 9-month follow-up. J Appl Oral Sci 26.
- Zidar A, Kristl J, Kocbek P, Zupančič Š (2021) Treatment challenges and delivery systems in immunomodulation and probiotic therapies for periodontitis. Expert Opin Drug Deliv 18(9): 1229–1244.
© 2022 Kimia Kelidari. This is an open access article distributed under the terms of the Creative Commons Attribution License , which permits unrestricted use, distribution, and build upon your work non-commercially.
 a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in
a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in 







.jpg)






























 Editorial Board Registrations
Editorial Board Registrations Submit your Article
Submit your Article Refer a Friend
Refer a Friend Advertise With Us
Advertise With Us
.jpg)






.jpg)














.bmp)
.jpg)
.png)
.jpg)










.jpg)






.png)

.png)



.png)






