- Submissions

Full Text
Modern Research in Dentistry
Azithromycin Induced Stevens Johnson Syndrome-A Rarity
Ashok Kumar and SK Roy Chowdhury*
Department of Oral and Maxillofacial Surgery, Army Dental Centre (Research and Referral), Delhi Cantt, New Delhi, 110010, India
*Corresponding author: SK Roy Chowdhury, Department of Oral and Maxillofacial Surgery, Army Dental Centre (Research and Referral), Delhi Cantt, New Delhi, 110010, India
Submission: November 22, 2021;Published: January 28, 2022

ISSN:2637-7764Volume7 Issue1
Abstract
Stevens Johnson Syndrome is a mucocutaneous immunological reaction reported commonly due to use of drugs such as sulfonamides, oxicam NSAIDs, allopurinol, aromatic anticonvulsants and anti-HIV drugs like nevirapine. The severity depends on the extent of body surface area involved and is called SJS/ TEN or TEN accordingly. The treatment of SJS is cessation of the causative drug, systemic steroids and supportive care. SJS due to azithromycin is a very rare occurrence and hence we report such a case which was successfully treated at our hospital.
Keywords: Sulfonamides; Allopurinol; Anticonvulsants
Abbreviations:SJS: Stevens Johnson Syndrome; TEN: Toxic Epidermal Necrolysis; Azithromycin
Introduction
Stevens-Johnson syndrome (SJS) and toxic epidermal necrolysis (TEN) are rare immunemediated severe cutaneous adverse reactions [1]. The incidence rate is approximately 0.05 to 2 persons per million populations per year. Drugs are the most commonly implicated in 95% of cases [1]. In a multicentric study conducted at Government Medical College, Bhavnagar, Gujarat, antimicrobials (50%), NSAIDs (22.41%), and antiepileptics (18.96%) were the most commonly associated groups. Nevirapine (28.12%) was the most common drug [2]. The incidence is higher in HIV positive individuals [3]. Clinically, SJS and TEN present with polymorphic lesions as erythematous macules, papules, plaques, vesicles, and bullae with predilection to distal extremities and a positive Nikolsky’s sign. “Target” lesion with bull’s eye appearance is characteristic of SJS and TEN. Oral, genital, and conjunctival mucosa is often involved in the form of erosions or ulcerations [4]. Azithromycin is a widely used drug and is considered safe. Gastrointestinal symptoms and reversible hearing loss are common adverse effects. Potentially serious ADRs like angioedema and cholestatic jaundice occur in about 1% of patients. Serious Fixed Drug Eruptions like SJS/TEN are extremely rare [5]. We report a case of SJS due to azithromycin in a middle aged previously healthy male who was successfully treated at our hospital.
Case Report
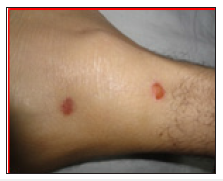
A 43-year-old male presented our hospital with swollen and cracked lips, redness of both eyes with profuse lacrimation and difficult vision and numerous skin rashes of 2 days duration. He was treated with Azithromycin by a medical officer in a clinic for sore throat. Clinical examination revealed numerous ulcerative lesions over buccal mucosa and lips and were most pronounced in the anterior parts of the mouth (Figure 1). Skin showed erythematous maculopapular lesions with evidence of blistering and ulceration with a positive Nikolsky’s sign. Classic target or iris lesions were seen in a symmetrical distribution (Figure 2). He had bilateral conjunctival chemosis with corneal sparing. He was diagnosed to have SJS based on clinical features and Azithromycin was stopped immediately. Oral prednisolone 1.0 mg/kg/day was given for 7 days and tapered over the next 21 days. Tetracycline and chlorhexidine mouth washes were given thrice daily and topical lignocaine was used for pain relief. Conjunctival involvement required topical steroids and cyclosporine. RT feeds were given till the time he could start tolerating oral feeds. The patient showed significant improvement with complete disappearance of rashes by 4th week of treatment.
Figure 1: a, b and c showing labial & buccal lesions and conjunctival chemosis.
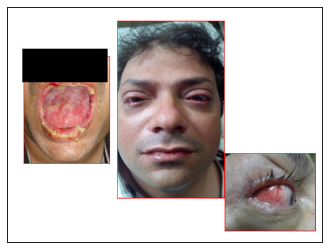
Figure 2:Target lesions on skin.
Discussion
Stevens Johnson Syndrome is an uncommon FDE and is rare with a safe drug like azithromycin [5]. It forms a part of a spectrum of severe cutaneous reactions (SCAR) which affect skin and mucous membranes with SJS at one end to SGS/TEN overlap and TEN at the other end. The distinction between SJS, SJS/TEN overlap, and TEN is based on the type of lesions and the amount of the body surface area involved. Blisters and erosions cover between 3% and 10% of the body in SJS, 11-30% in SJS/ TEN overlap, and over 30% in TEN. Erythema multiforme, which is also within the SCAR spectrum, differs in clinical pattern and etiology. Although both SJS and TEN can be caused by infections, they are often adverse effects of medications [4]. Drugs that are commonly associated are sulfonamides, beta lactams, sedatives like barbiturates, anticonvulsants (phenytoin and lamotrigine), allopurinol and ART drugs (Nevirapine) [2]. It is an immunologically mediated disease and the cytolytic protein granulysin, produced by drug specific CD8+ T cells and natural killer (NK) cells, has been identified as the most important factor for the epidermal destruction [6], the concentration of which in blisters correlates directly with prognosis [7]. It usually begins with fever, sore throat, and fatigue and evolves to skin and mucosal involvement with typical erythematous maculopapular vesicles, and bullae with predilection to distal extremities and a positive Nikolsky’s sign and “target” lesions with bull’s eye appearance [4]. The course can be fulminant with multi organ failure and death in about 5% of patients. Corneal scarring and staphylococcal septicemia are known [8]. Treatment is primarily supportive and symptomatic with special attention to airway and hemodynamic stability, fluid status, wound/burn care, and pain control. Immunomodulation with corticosteroids, cyclophosphamide and immunoglobulin have been advocated but are controversial. Oral lesions are managed with mouthwashes. Topical anesthetics are useful in reducing pain and allowing the patient to take in fluids [9].
Conclusion
SJS is a rare adverse reaction to azithromycin usage. The syndrome is often associated with significant morbidity and is lethal in about 5% patients, if not identified early and requires supportive measures and immunomodulatory therapy with steroids though controversial, after prompt stoppage of causative drug. Diagnosis is clinical and hence a high index of suspicion is necessary to identify SJS due to drugs that seldom lead to such severe cutaneous reactions.
References
- Sharma VK, Sethuraman G (1996) Adverse cutaneous reactions to drugs: An overview. J Postgrad Med 42(1): 15-22.
- Barvaliya M, Sanmukhani J, Patel T, Paliwal N, Shah H, et al. (2011) Drug-induced Stevens-Johnson syndrome (SJS), toxic epidermal necrolysis (TEN), and SJS-TEN overlap: A multicentric retrospective study. J Postgrad Med 57(2): 115-119.
- Khambaty MM, Hsu SS (2010) Dermatology of the patient with HIV. Emerg Med Clin North Am 28(2): 355-368.
- Mockenhaupt M (2009) Severe drug-induced skin reactions: Clinical pattern, diagnostics and therapy. J Dtsch Dermatol Ges 7(2): 142-160.
- Das A, Sancheti K, Podder I, Das NK (2016) Azithromycin induced bullous fixed drug eruption. Indian J Pharmacol 48(1): 83-85.
- Nassif A, Moslehi H, Le Gouvello S, Bensussan A, Wolkenstein P, et al. (2004) Toxic epidermal necrolysis: effector cells are drug-specific cytotoxic T cells. J Allergy Clin Immunol 114(5): 1209-1215.
- Chung WH, Hung SI, Yang JY, Su SC, Huang SP, et al. (2008) Granulysin is a key mediator for disseminated keratinocyte death in Stevens–Johnson syndrome and toxic epidermal necrolysis. Nat Med 14(12): 1343-1350.
- Raksha MP, Marfatia YS, (2008) Clinical study of cutaneous drug eruptions in 200 patients. Indian Journal of Dermatology, Venereology and Leprology 74(4): 430.
- Khalili B, Bahna SL (2006) Pathogenesis and recent therapeutic trends in Stevens-Johnson syndrome and toxic epidermal necrolysis. Ann Allergy Asthma Immunol 97(3): 272-280.
© 2022 SK Roy Chowdhury. This is an open access article distributed under the terms of the Creative Commons Attribution License , which permits unrestricted use, distribution, and build upon your work non-commercially.
 a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in
a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in 







.jpg)






























 Editorial Board Registrations
Editorial Board Registrations Submit your Article
Submit your Article Refer a Friend
Refer a Friend Advertise With Us
Advertise With Us
.jpg)






.jpg)














.bmp)
.jpg)
.png)
.jpg)










.jpg)






.png)

.png)



.png)






