- Submissions

Full Text
Modern Research in Dentistry
Effect of Two Dopamine Application Methods on the Immediate Composite-Dentin Microtensile Bond Strength
Zahra Khamverdi1*, Pedram Pakzamir2, Zeinab Mohamadi1, Mohammad Parsa Lohrasbi3 and Farzaneh Shirani4
1Dental Research Center, Department of Operative Dentistry, Iran
2Faculty of Dentistry, Iran
3Dental School, Silesia Medical University, Poland
4Dental Material Research Center, Isfahan University of Medical Sciences, Iran
*Corresponding author: Zahra Khamverdi, Dental Material Research Center, Department of Operative Dentistry, School of Dentistry, Isfahan University of Medical Sciences, Isfahan, Iran
Submission: December 12, 2023;Published: January 12, 2024

ISSN:2637-7764Volume8 Issue1
Abstract
Purpose: The improvement of dentin-composite bond strength has always been a concern in restorative
dentistry. Few studies have researched the effect of dopamine on the strength of composite-dentin bonds.
The aim of this study is to evaluate the effect of two dopamine application methods on microtensile bond
strength between composite resin and dentin.
Materials and Methods: The enamel of occlusal surfaces of thirty human third molars were removed
and undivided into three equal groups after acid etching according to dentin surface treatment as follows:
G1: Control group (without dentin treatment); G2: treated with immersion in dopamine solution for a
duration of 24h; and G3: treated with dopamine solution (30s) by scrubbing. All of the samples were
bonded with light-cured fifth-generation dentin bonding and built up by using a light-cured composite
resin. Two 1-mm bar-shaped sections of each tooth were prepared (n=15). The bond strength values of
the samples were recorded by a universal machine with 0.5mm/min speed. Data were analyzed using
one-way ANOVA and post-hoc Tukey tests (α=0.05).
Result: The mean and standard deviation of microtensile bond strength values in groups 1-3 were
11.64±1.22, 12.95±1.1, and 14.74±2.6, respectively. There was a significant, statistically, difference
between G1 and G2, as well as G1 and G3 (P<0.0) but significant no difference was observed between G2
and G3 (P > 0.05).
Conclusion: The results showed treatment of the dentin surface with dopamine increases the immediate
microtensile bond strength of composite resin to dentin. The dopamine scrubbing strategy is more
effective than the immersion method.
Keywords: Microtensile, Dopamine, Composite resin, Dentin, Bond strength
Abbreviations: PDA: Polydopamine; MAP’s: Mussel Adhesive Proteins; SEM: Scanning Electron Microscope
Introduction
Composite resin restorations are the preferred option for direct restoration of both anterior and posterior teeth. The long-term bond strength of composite resins to enamel have provided various clinical successes. Unfortunately, dentin has a heterogeneous nature which complicates the effective bonding. Many strategies such as ethanol wet bonding, matrix metalloproteinase inhibitors, collagen cross-linkers, and dentinal remineralization have been suggested to improve the physio-chemical resistance of the hybrid layer and the durability of the composite-dentin interface bond [1].
Polydopamine (PDA) is an organic molecule that includes both catechol and amine groups. PDA is formed spontaneously due to dopamine oxidative polymerization in alkaline solutions (pH>7.5). An important factor involved in the adhesion of PDA is a group of proteins called mussel adhesive proteins (MAP’s). These proteins are secreted from the tiny strands attached to the mussel and cause the formation of a protein plaque on the mussel’s surface.
MAP’s contain 3,4-dihydroxy-L-phenylalanine (DOPA) and lysine. The function of this substance is not yet fully understood, but its adhesive properties seem to be associated with dopamine, which is formed on hard surfaces as PDA [2]. Yang et al. [3] conducted an experimental study on the adhesion of the PDA membrane to polymer, glass, and iron surfaces. They showed that dopamine could bond to all surfaces, polymerize spontaneously, and form a thin hydrophilic layer. This study indicated that dopamine could bond two hard surfaces (e.g. aluminum and glass) but was not effective in surfaces of other materials [4].
The positive effects of MAP’s in dentistry as having the antimicrobial property [5], facilitating the formation of hydroxyapatite crystals parallel to the PDA layers [6], reducing the matrix metallopeptidase activity and impairing dentinal collagens [7], and increasing dentinal remineralization and sealing dentinal tubules by hydroxyapatite crystals [8] have been reported. In addition, low cost, availability, as well as easy preparation and application of this component are proper options in adhesive dentistry.
Due to lack of sufficient information, the clinical uses of PDA in adhesive dentistry, this study carried out to evaluate the effect of PDA application as a primer on the composite-dentin microtensile bond strength by using the etch-and-rinse bonding system. The null hypothesis was that the treatment of the dentinal surfaces with PDA has no effect on the resin composite microtensile bond strength to dentin.
Materials and Methods
Production of dopamine solution
To prepare buffer for solving dopamin, 10mM Tris buffer with a pH>8.5 was used. According to the Horizon Discovery protocol, first, 12.1g Tris base powder (Cinnagen, Tehran, Iran) was dissolved in 80mL distilled water, and the solution pH was adjusted to 8.5 by adding HCI. Addition of HCL was performed slowly to prevent high temperature. Then, the solution was reach to 100mL by adding distilled water and was filtered by a 0.22μm sterile filter, which yielded a 1mM solution. To obtain a 100mL 10-mM Tris buffer solution, 1mL of the prepared solution was added to 99mL distilled water. Then, to prepare 100mL 2mg/ml dopamine solution, 0.2g dopamine hydrochloride powder (H8502, Sigma-Aldrich, USA) was added to 80mL Tris buffer solution, and the solution volume was increased to 100mL.
Sample preparation
A total of 30 third molars, without caries, crack, hypoplasia, and
restoration, were extracted due to periodontal problems during
the past 3 months and were collected and stored in 10% formalin
(Dr. Mojallali, Kaveh Industrial City, Iran) at room temperature.
The collected samples were fixed in the acrylic blocks and their
occlusal surface enamel was removed by an orthodontic trimmer
(Renfert, Hilzingen, Germany) under cool water flow perpendicular
to the longitudinal axis of the tooth to obtain a flat dentinal surface
without any enamel. The surfaces were then polished by 400-800
grit discs using a low-speed handpiece under water flow for 15
seconds to create a uniform smear layer. All teeth were etched 37%
phosphoric acid gel (South Jordan, USA, Ultra etch, Ultra dent) for
15 seconds, and were, then, rinsed for 15 seconds. The samples
were randomly divided into three groups (n=10) according to type
of dentin treatment as follows:
A. In the group 1(control), the dentinal surface of the samples
was prepared for bonding to composite, based on the
manufacturer’s instructions withouth dentin surface
treatment.
B. In group 2 (immersion), the samples were immediately
immersed in 2mg/ml dopamine solution after etching for 24 h,
then were rinsed under water stream for 1 minm, dried gently
and bonded using light cured fifth-generation dentin bonding
system (Adper Single bond 2, 3M EPSE, St.Paul, USA) following
to the manufacturer’s instructions similar to.
C. In group 3, immediately after etching, the dentinal surface
was impregnated with 2mg/mL dopamine by a microbrush
2 times, each time for 30 seconds, then was dried gently. For
bonding of all samples, a light curing fifth-generation dentin
bonding system (Adper Single bond 2, 3M EPSE, St.Paul, USA)
was applied to the surface of the samples in two layers by a
microbrush for15 seconds and light cured by a light-curing
unit (LED, Woodpecker, China) with 450mW/cm2 intensity
for 20 seconds from a distance of 1mm. Then, the light cured
composite resin (Filtek Z250, 3M ESPE, St.Paul, MN, USA) was
bonded to the dentin in 1mm layers, and each layer was cured
for 20 seconds. The samples were then placed in 37 °C water
for 24 h to complete the polymerization process.
Microtensile bond strength testing
Each sample was subjected under a water-cooled blade of a sectioning machine (Hamco Inc., Rochester, NY), one-millimeterthick sections were serially cut perpendicular to the long axis of each tooth, and two longitudinal cuts were made. Finally, sticks with uniform thickness were prepared, in which the tooth structure was placed in a side and the composite build-ups on each side (15 sticks for each group).
To assess the microtensile bond strength, a microtensile testing machine (Santam, Tehran, Iran) was used. The samples prepared were fixed with cyanoacrylate adhesive in the machine jig. The bonded cross-section of the samples was fixed perpendicular to the tensile force of the machine. The tensile force was applied to the composite-dentin interface with 5.0mm/min speed (ISO TR 1145) until fracture occurred. The values by the machine were recorded. The bond strength value of the samples was expressed in Mega Pascal (MPa), by dividing the load at failure (Newton) to the bonding surface area (mm2).
Fracture pattern and surface morphology assessment
To determine the fracture pattern, each sample was observed
using a stereomicroscope (SZX16, Olympus, Tokyo, Japan) at ×20
magnification and was scored as follows:
1) Adhesive: Fracture at composite-bonding or bonding-dentin
interface
2) Cohesive: Dentin or composite fracture
3) Mixed: A combination of adhesive and cohesive fracture
From each group, one sample was assessed by a Scanning Electron Microscope (SEM) to find the morphology of the fracture surface. the selected sample were placed on an aluminum stub by a conductive tape (bilateral carbon tapes) in a sputter coater (Desk sputter coater II, Nanostructured coatings co., Tehran, Iran) using the sputtering method, and were coated with palladium-gold alloy for 10 min. The samples were evaluated by an SEM (MIRA3 TESCAN, Brno, Czech Republic) and imaged at ×500, 1000, 2000 and 5000 magnifications.
Statistical analysis
The collected information was imported into the SPSS (version 26) IBM software. To determine the normality of the data in the study groups, the Kolmogorov-Smirnov test was used. The data was analyzed by one-way ANOVA and Tukey’s HSD tests. P<0.05 was considered significant.
Result
Table 1 shows the mean, standard deviation, and maximum and minimum bond strength values for the studied groups.
Table 1:Comparison of the micro bond strength for the studied groups.
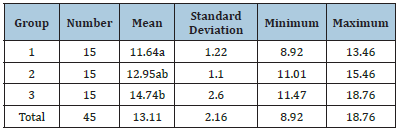
The mean values with similar letters were not significantly different from each other. The results of one-way ANOVA showed a significant difference in the bond strength mean among the three studied groups (p<0.05). There was a statistically significant difference between G1 and G2, as well as G1 and G3 (P<0.0), but significant difference was not observed between G2 and G3 (P>0.05).
The fracture pattern and surface morphology observation
The results of the frequency of the fracture pattern of the samples in each group by stereomicroscope are presented in Table 2.
Table 2:Fracture pattern of samples in the studied groups.
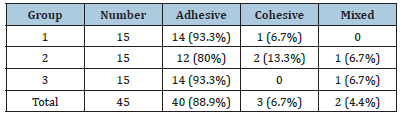
SEM observation of the fracture surfaces in the group1 showed dentinal tubules were open and bonding agent were extracted tubules. In the group 2, in which immersion in dopamine was performed for 24 hours, a large number of dentinal tubules were completely covered with dopamine, and the tubules were sealed by resin penetration. In the group 3, the orifices of the tubules were partially filled with dopamine, but not so much to be completely blocked, and the bonding agent could still penetrate into the dentinal tubules. Presence or absence of the bonding layer on the surface of samples are presented in (Figures 1-3).
Figure 1:SEM image of group 1. a: Sample surface of group 1 at ×500 magnification, b: Sample surface of group 1 at ×1000 magnification, c: Sample surface of group 1 at ×2000 magnification, d: Horizontal image of the fractured edge of samples in group 1 at ×5000 magnification.

Figure 2:SEM images of group 2. a: Sample surface of group 2 at ×500 magnification, b: Sample surface of group 2 at ×1000 magnification, c: Sample surface of group 2 at ×2000 magnification, d: Horizontal image of the fracture edge of samples in group 2 at ×5000 magnification.

Figure 3:SEM images of group 3. a: Sample surface of group 3 at ×500 magnification, b: Sample surface of group 3 at ×1000 magnification, c: Sample surface of group 3 at ×2000 magnification, d: Horizontal image of the fracture edge of samples in group 3 at ×5000 magnification.

Discussion
Many studies have been evaluated on the remarkable properties of PDA in increasing the adhesion between different surfaces such as metals and polymers [9]. It has been proven that catechol groups in dopamine cause the adhesion of mussels to different surfaces like stones, metals, and glass by forming hydrogen bonds and metalligand complexes [10]. Therefore, it may be benefit in adhesive dentistry. Since few studies have ever investigated the effect of dopamine on the composite-dentin microtensile bond strength, the current study aimed to evalute the effect of dopamine on the composite-dentin interface microtensile bond strength.
Nowadays, dopamine is available as dopamine hydrochloride powder. PDA can be produced in various conditions, but the most frequently used protocol is oxidation in solution [11]. PDA is spontaneously formed in a single phase via pH-based polymerization and dopamine-hydrochloride oxidation in the alkaline solution (pH>7.5). The formation of the PDA layer requires that the concentration of dopamine monomers must be more than 2mg/mL. Despite the easy formation of PDA, its molecular mechanism is not yet known completely [12]. In this study, 2mg/ mL dopamine-hydrochloride was used in 10mM Tris buffer with a pH of 8.5, which was presented by Lee et al. [9] as a protocol for creating a PDA layer with 50nm thickness [13].
To determine the bonding strength difference between groups, the microtensile bond strength test was used. It has been proven that the microtensile bond strength test has a higher differentiation ability than the shear bond strength test in assessing the compositedentin bonding. The microtensile bond strength test is currently used as a multi-purpose and standard test for the evaluation of bonding ability [14]. Based on the results obtained in this study, the scrub groups showed considerable higher microtensile bond strength than the group with no dopamine application. Dentinal adhesion is firstly dependent on the penetration of adhesive monomers into the network of collagen fibers open to the material via acid etching [15]. Li et al. [10] conducted a study on the surface changes of fiber posts using dopamine polymerization at the curing agent-post interface and showed that the dopamine-impregnated surface has a lesser roughness [16]. It has been shown that surfaces with higher roughness have more surface defects and stressaccumulated areas into which the adhesive agent may not fully penetrate and can provide a higher thickness and consequently cause a poorer bond strength [17].
Li [11] also reported that the contact angle of water and dopamine-impregnated post surface was, greatly, reduced [17], and since the bonding agent used in this study contains water in its composition, it can have a better wetting on the dopamineimpregnated surface, and therefore spread better, and create a stronger bonding surface.
The decomposition of exposed collagen fibers is one of the factors affecting the reduced composite-dentin bond strength. Studies have shown that the catechol group in dopamine has an inhibitory effect against bacterial collagenases. Owing to the development of collation-metal bonding, the catechol group in dopamine collates to the calcium ion (which is necessary for the activity of collagenase bacteria) and inhibits the activity of bacterial collagenases. The inhibitory effect on collagenization has been observed with the EDTA solutions as well.
Moreover, dopamine can cross-link with dentin by establishing a covalent bond between the catechol group and the amine group of collagen fibers. This bond hardens the collagen fibers and inhibits the decomposition of its triple helix structure [18]. Studies have reported the effect of cross-linkers such as proanthocyanidin on the increased composite-dentin bond strength [19]. The compositedentin bond strength depends not only on the decomposition of collagen fibers, but also on the humidity rate of the dentinal surface. Owing to its water-proof property, dopamine can overcome the hydration repulsive forces on the dentinal surface and create a stronger bond [20]. Fang et al. [8] investigated the effect of MAP on the composite-dentin bond [21] and Chen et al. [16] studied the effect of dopamine on the bond strength of intracanal glass fiber post and reported similar results [21].
The results of SEM analysis of the dentinal surface showed the closure of dentinal tubules by resin tags in 2 and 3 groups and confirmed microtensile bond strength results. Fang et al. [8] also found similar results [21]. Although the bond strength was increased with dopamine immersion, no significant difference was observed in this study between the group where no dopamine was used and the group where dopamine was applied with the immersion method.
As depicted in SEM images, this could be associated with the excessive thickness of PDA formed on the dentinal surface during 24h by the immersion method, which could inhibit the increased bond strength compared to the scrub group. In addition, even though dopamine immersion increased the wettability of the dentinal surface for the adhesive application, it has most likely blocked the penetration of adhesives into the dentinal tubules, and therefore has only exerted limited effect on the immediate microtensile bond strength. It seems that the material is set on the surface with a better thickness in the scrub group, while it has a less positive effect when the immersion time is higher. Further studies are required to explore the effect of concentration and application time of dopamine. Based on the results of this study, it can be assumed that the use of 2mg/ml dopamine as a scrub for 60 seconds significantly enhances the composite-dentin microtensile bond strength.
Li et al. [10] evaluated the application of a mussel-inspired molecule in dentin bonding. They used a combination of Dopamine Methacrylate (DMA) and Dimethyl Sulfoxide (DMSO) in different concentrations as a primer on the acid-etched dentinal surfaces before application of the Adper Single Bond 2. They showed that the application of 1mMDMA/DMSO solution for 60 seconds increased and protected the dentinal bond strength as a bridge connecting the collagen fibrils and adhesive [1]. In this study, the immediate bond strength did not increase with the application of dopamine, when compared to the control group, but bond durability was greatly influenced by the application of 1mM DMA/DMSO solution, which ensued the significant survival of the bond in comparison to the control group. The difference between the immediate bond strength of the present study and that of the study of Li can be due to the different concentrations and composition of dopamine mixtures used. However, further studies are required to study the effect of aging on the survival of the bond created by dopamine in this study.
Despite the easy preparation and low cost of dopamine used in this study, one of the limitations of using this composition of dopamine is the black color of the solution, which causes noticeable discoloration on the dentinal surface during immersion before application of the composite. However, in the scrub method, since the time of solution application was not more than 60 seconds, no significant discoloration appeared on the dentinal surface. Further quantitative studies are suggested to measure color parameters in this regard and assess the effect of time on discoloration.
As per manufacturer instructions of the adhesive agent used in this study, 2 layers of the etch-and-rinse adhesive agent, one for priming and one for adhesion, should be applied before composite resin placement. The possibility of application of only one layer of the adhesive agent after dopamine scrubbing should be explored in future studies in order to reduce the clinical application time of dopamine.
Conclusion
The results showed treatment of the dentin surface with dopamine increases the immediate microtensile bond strength of composite resin to dentin. The dopamine scrubbing strategy is more effective than the immersion method..
Acknowledgment
The authors would like to thank the Dental research Center, Hamadan University of Medical Sciences, Iran, for their support.
Financial Support and Sponsorship
Hamadan University of Medical Sciences.
References
- Aguiar T, Vidal C, Phansalkar R, Todorova I, Napolitano J, et al. (2014) Dentin biomodification potential depends on polyphenol source. Journal of Dental Research 93(4): 417-422.
- Armstrong S, Breschi L, Özcan M, Pfefferkorn F, Ferrari M, et al. (2017) Academy of Dental Materials guidance on in vitro testing of dental composite bonding effectiveness to dentin/enamel using micro-tensile bond strength (μTBS) approach. Dental Materials 33(2): 133-143.
- Yang FK, Zhao B (2011) Adhesion properties of self-polymerized dopamine thin film. The Open Surface Science Journal 3(1): 115-122.
- Blum IR, Hafiana K, Curtis A, Barbour ME, Attin T, et al. (2012) The effect of surface conditioning on the bond strength of resin composite to amalgam. Journal of Dentistry 40(1): 15-21.
- Cheng W, Zeng X, Chen H, Li Z, Zeng W, et al. (2019) Versatile polydopamine platforms: synthesis and promising applications for surface modification and advanced nanomedicine. Acs Nano 13(8): 8537-8565.
- Demarco FF, Collares K, Correa MB, Cenci MS, Moraes RRD, et al. (2017) Should my composite restorations last forever? Why are they failing? Brazilian Oral Research 31(Suppl): e56.
- Epasinghe DJ, Yiu CKY, Burrow MF, Hiraishi N, Tay FR (2013) The inhibitory effect of proanthocyanidin on soluble and collagen-bound proteases. Journal of Dentistry 41(9): 832-839.
- Fang H, Li QL, Han M, Mei ML, Chu CH (2017) Anti-proteolytic property and bonding durability of mussel adhesive protein-modified dentin adhesive interface. Dental Materials 33(10): 1075-1083.
- Lee H, Lee BP, Messersmith PB (2007) A reversible wet/dry adhesive inspired by mussels and geckos. Nature 448(7151): 338-341.
- Li K, Sun Y, Tsoi JKH, Yiu CKY (2020) The application of mussel-inspired molecule in dentin bonding. Journal of Dentistry 99: 103404.
- Li Y, Chen Q, Yi M, Zhou X, Wang X, et al. (2013) Effect of surface modification of fiber post using dopamine polymerization on interfacial adhesion with core resin. Applied Surface Science 274: 248-254.
- Palladino P, Bettazzi F, Scarano S (2019) Polydopamine: surface coating molecular imprinting, and electrochemistry-successful applications and future perspectives in (bio) analysis. Analytical and Bioanalytical Chemistry 411(19): 4327-4338.
- Bernsmann F, Ball V, Addiego F, Ponche A, Michel M, et al. (2011) Dopamine- melanin film deposition depends on the used oxidant and buffer solution. Langmuir 27(6): 2819-2825.
- Perdigao J, Swift E, Walter R (2019) Fundamental concepts of enamel and dentin adhesion. Sturdevant’s Art and Science of Operative Dentistry, pp. 114-140.
- Petrone L, Kumar A, Sutanto CN, Patil NJ, Kannan S, et al. (2015) Mussel adhesion is dictated by time-regulated secretion and molecular conformation of mussel adhesive proteins. Nature communications 6: 1-12.
- Chen Q, Wei XY, Yi M, Bai YY, Cai Q, et al. (2015) Effect on the bond strengths of glass fiber posts functionalized with polydopamine after etching with hydrogen peroxide. Dental Materials Journal 34(6): 740-745.
- Ryu J, Ku SH, Lee H, Park CB (2010) Mussel‐inspired polydopamine coating as a universal route to hydroxyapatite crystallization. Advanced Functional Materials 20(13): 2132-2139.
- Silverman HG, Roberto FF (2007) Understanding marine mussel adhesion. Marine Biotechnology 9(6): 661-681.
- Thompson JM, Agee K, Sidow SJ, McNally K, Lindsey K, et al. (2012) Inhibition of endogenous dentin matrix metalloproteinases by ethylenediaminetetraacetic acid. Journal of Endodontics 38(1): 62-65.
- Van Meerbeek B, Yoshihara K, Yoshida Y, Mine A, De Munck J, et al. (2011) State of the art of self-etch adhesives. Dental materials 27(1): 17-28.
- Xu Q, Li Q, Chen J, Zhang W, Wu X, et al. (2015) Effect of dopamine on the activity of matrix metalloproteinases and degradation of dentin collagen. Chinese Journal of Stomatology 50(3): 186-189.
© 2024 Zahra Khamverdi. This is an open access article distributed under the terms of the Creative Commons Attribution License , which permits unrestricted use, distribution, and build upon your work non-commercially.
 a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in
a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in 







.jpg)






























 Editorial Board Registrations
Editorial Board Registrations Submit your Article
Submit your Article Refer a Friend
Refer a Friend Advertise With Us
Advertise With Us
.jpg)






.jpg)














.bmp)
.jpg)
.png)
.jpg)










.jpg)






.png)

.png)



.png)






