- Submissions

Full Text
Modern Concepts & Developments in Agronomy
Study on the Influence of Climatic Factors in Greenhouses on the Growth, Yield, and Biochemical Attributes of Tomatoes Grown in the Soilless System During the Summer Season
Adnan Arshad1, Chan Sovorn1, Elena Maria Draghici1*, Ionut Ovidiu Jerca2 and Naeem Iqbal3
1Bioengineering of Horticultural and Viticultural systems Department, Faculty of Horticulture, Romania
1Environment and Land Reclamation Department, Faculty of Land Reclamation and Environmental Engineering, Romania
1Department of Botany, Faculty of Life Science, Pakistan
*Corresponding author:Elena Maria Draghici, Bioengineering of Horticultural and Viticultural systems Department, Faculty of Horticulture, University of Agronomic Sciences and Veterinary Medicine of Bucharest 011464, City, Bucharest, Romania
Submission: March 25, 2024;Published: April 12, 2024

ISSN 2637-7659Volume14 Issue 2
Abstract
The cultivation of cherry tomatoes during the summer season poses significant challenges concerning growth, yield, and development. Cultivating cherry tomatoes while upholding optimal climatic conditions throughout the scorching summer months requires a comprehensive assessment. Experimental studies were carried out to investigate the potential impact of greenhouse climate on cherry tomatoes, “Cheramy F1” (Solanum Lycopersicon var. cerasiforme) in the soilless system during the summer season in two consecutive years, 2022 and 2023. The experiments were carried out inside the greenhouse at the University of Agronomic Sciences and Veterinary Medicine of Bucharest (44.4710° N, 26.0656° E). The relationship between the plants’ growth and applied microclimatic conditions was evaluated based on their impact on changes in plant total height, growth rate, stem diameter, and total leaf count. The productivity performance of plants has been observed based on data available regarding the length of inflorescence, total mass per inflorescence, average fruit mass per inflorescence, fruit firmness, fruit keeping days, and fruit biochemical components. The growth attributes significantly varied on different observation dates in relation to available greenhouse conditions. Significant interactions between available greenhouse conditions and certain growth attributes have been observed. The greenhouse experienced fluctuations in conditions during the vegetative and reproductive stages - 23.16 °C and 20.26 °C and light and CO2 - 468.46 °C and 356.02 °C W/m2, - 479.27ppm and 446.06ppm a, appeared with maximum growth and productivity influence during the months of July and august. The results reveal both positive and negative linear correlations between greenhouse factors and productivity attributes. Awareness of climate impacts enables growers to adjust conditions, preventing stressors and enhancing crop resilience and productivity,” emphasizes the critical role of environmental control and grower knowledge in achieving successful cherry tomato cultivation in greenhouses. Co-relation and analysis of variance were used to statistically analyze the presented results at a confidence level of p < 0.05.
Keywords:
Greenhouse cultivation; Summer grown-Cherry tomatoes; Growth; Productivity; Biochemical compositionIntroduction
Cultivating tomatoes in a greenhouse involves a meticulous approach, considering several essential factors: growing substrate, nutrient availability, optimum ranges of greenhouse climatic parameters, and the cultivar chosen. In today’s agricultural landscape, ensuring a sustainable environment for plant growth has become essential for horticulturalists to mitigate the impact of unpredictable climate shifts. Different plant species have evolved to thrive in particular environmental conditions, including variations in climate and air chemistry [1,2].
These variations can significantly affect the growth, development, and overall health of plants. Factors such as temperature, light intensity, carbon dioxide levels, and air circulation directly influence plant development and metabolic processes. Growing plants in a greenhouse utilizing soilless systems offers an alternative method to open-field cultivation. The cultivation of vegetables in greenhouses plays an increasingly pivotal role in producing food for humans [3]. Cherry tomato holds significant importance as a most popular vegetable fruit, predominantly cultivated within greenhouses. In Europe, the average annual total production of tomatoes reached 3.2 million tons in 2022 [4,5]. “The rich nutritional content of cherry tomatoes is well known, providing essential nutrients (Brix, Nitrate), vitamins (A and C), minerals (potassium, calcium, and phosphorus folate), and antioxidants [6-8]. Tomatoes contain lycopene and bioflavonoids, which are effective in combating cancer and promoting cardiovascular health by acting as antioxidants. Their lycopene content supports skin health, energy restoration, and lower blood pressure, whether consumed raw or processed [9]. Understanding greenhouse climate conditions is essential for sustainable plant growth because it allows growers to create and maintain optimal environments for their crops [10,11]. In the present research-based study, we investigated the growth, yield, and biochemical response of a summer-grown local cultivar of cherry tomatoes, Cheramy F1 to greenhouse microclimate conditions in a soilless system.
Cherry tomatoes thrive in diverse growing substrates and environments due to their adaptive nature, exhibiting resilience and versatility that make them suitable for various cultivation methods. Nowadays, the practice of cultivating tomatoes without soil, using peat, perlite, or Coco coir, is commonly observed in greenhouse settings [12]. Soilless systems typically employ drip irrigation or other efficient watering methods, reducing water waste and promoting water conservation. Water and salinity stress have been found to affect the composition of cherry tomato fruits by increasing the concentration of sugars and acids within them, consequently impacting both taste and nutritional profile [13]. The tomato plant’s production and accumulation of energy through photosynthesis, later translocated to developing fruits, are significantly influenced by temperature. The growth and developmental processes of cherry tomatoes are significantly impacted by elevated temperatures, leading to a significant reduction in plant yield [14]. During the germination stage, temperatures between 16 °C and 29 °C have been reported as most conducive. As growth advances, the optimal temperature range shifts from 22 °C to 26 °C. Subsequently, during the fruit set stage, temperatures ranging from 14 °C to 24 °C have been identified as ideal [15-17]. High temperatures exceeding 30 °C cause a notable rise in evapotranspiration rates, leading to decreased carbohydrate biosynthesis allocated to assimilation and sweetness in plants [18]. Consequently, there is an inhibition of lycopene biosynthesis, prompting a shift in fruit coloration towards yellow or orange, as noted [19]. Current research has determined the ideal temperature ranges for maximizing the growth and productivity of tomato plants. Temperature and carbon dioxide have been reported as two crucial climate variables that reduce the yield potential of cherry tomatoes [20,21]. In current times, greenhouse vegetables are cultivated utilizing carbon dioxide (CO2) as a gaseous fertilizer, adopted due to CO2’s ability to enhance plant resilience to climate change stress, as indicated by various studies [22,23]. Increasing carbon dioxide levels can boost the net photosynthetic rates of plants, thereby enhancing their growth and yield [24].
Environmental conditions during pre- and post-harvest stages also significantly influence the quality traits of cherry tomatoes. Increased harvest losses in cherry tomato cultivation are often attributed to a lack of knowledge and methodology in cultivation practices [25]. The quality of a product can be shaped by various factors, which can be categorized into two main groups: intrinsic factors, including color, shape, and absence of defects, and internal attributes such as texture, sweetness, acidity, aroma, flavor, shelf life, and nutritional value [26]. Previous studies have reported that the quality of the product is influenced by both external conditions and crop management during the pre-harvest period [21,27]. Extending the shelf life of tomatoes significantly relies on greenhouse management and the grower’s understanding [28]. Seasonal fluctuations in sunlight exposure can influence the levels of soluble sugars and antioxidants found in tomatoes cultivated in greenhouses. Tomatoes grown in greenhouses may exhibit varying levels of antioxidants since they absorb less UV light compared to those grown in fields, as noted by [29]. Insufficient light levels impede pigment synthesis, resulting in irregular plant coloring. High and low light levels significantly influence carotenoids and ascorbic acid [30].
Material and Method
Experimental site and greenhouse management
Experimental studies were conducted at the University of Agronomic Sciences and Veterinary Medicine of Bucharest over an extended period from May to August in both 2022 and 2023 to assess the response of cherry tomatoes (Cheramy F1) to greenhouse conditions. The research was carried out inside a Venlo Glass greenhouse, a unit of the Research Center for Quality Control of Horticultural Products. The greenhouse structure consisted of hot-dipped galvanized steel, aluminum system profiles for external cladding, and glass covering, with a cultivating gutter height of 5.30 meters above the soil level. The growing compartment spanned 160.00m² within a total covered area of 2,752.00m². Advanced data recording devices, lighting, drip irrigation, and ventilation systems were also installed in the greenhouse to measure extreme summer climatic conditions.
Biological material and procedure
Cherry tomato seeds (Solanum Lycopersicon var. cerasiforme) of the “Cheramy F1” variety were sown in plastic plug trays with cells 3.5cm deep and wide, filled with disinfected coco peat. After a 10-day emergence, seedlings were transplanted into perlite pots, and after that, 45-day-old seedlings with the first inflorescence appeared were transferred to the experimental compartment. White cubes filled with sterilized hydroponic perlite (2mm) were prepared as the planting medium and positioned on coconut slabs within gutters (Figure 1). Various concentrations of nutrient solution were administered at different growth stages: during seedling development with an Electrical Conductivity (Eco) of 1.5, upon emergence of the 1st and 2nd inflorescences with an Eco of 2.3, and between the 3rd and 6th inflorescences with an Eco ranging from 2.8 to 3.0m Siemens, all maintained at a constant pH of 5.5. Irrigation, both in cubes and slabs, was administered 10-12 times daily during the fruiting phase, with each plant receiving 100- 150ml of nutrient solution. Plants were spaced at 33cm intervals with a density of 3 plants per square meter, and a gap of 1.5cm was maintained between the stationary benches.
Figure 1:(a) The growth of seedlings in white cubes. (b) Interior perspective of the experimental compartment within the greenhouse.

Experimental design and Statistical analysis
Three experimental rows were selected for biochemical, physical, and growth analysis, with three random plants chosen from each row (designated as p1, p2, and p3 for Row 1, Row 2, and Row 3, respectively), following the method outlined in [31]. The average growth rate was determined by dividing the measured data by the number of days between each recording. Productivity parameter graphs were generated using MS Excel (365) and Jamvoi software (2.4). Subsequently, Pearson co-relation and Analysis of Variance (ANOVA) were performed, accompanied by the Shapiro- Wilk normality test and Levene’s test for homogeneity of variances, with a significance level set at p < 0.05.
Data Collection
Growth attributes
The activity of data collection was started during the flowering and early fruit setting stages, lasting from May to September with variable intervals during the years 2022 and 2023, and is averaged over two years. The primary aim was to evaluate the growth rate of plants during the maturity stage. Plant height (cm) was measured from the base to the terminal growing point of the main stem using a measuring tape, with the average of three plants per row reported. Plant growth was assessed by dividing measured height by days between measurements. The length (Ls) and width (Lw) of each marked leaf were recorded, and leaf area was calculated using a specified formula. Leaf selection aimed to correlate leaf area with inflorescence productivity, examining leaves below each inflorescence in rows (Inflorescence 1 to 14) on each observation date. The total leaf count per observation date was determined by averaging counts across three plants per row. The stem diameter was measured with a digital caliper and expressed in millimeters. The average distance between two leaves and two inflorescences was measured with a measuring tape and expressed in centimeters. Leaf fresh and dry weights were obtained by collecting representative leaves, chopping them into small pieces, and weighing them before and after drying. The samples were dried in an oven at 105 °C for 24 hours with a 50% air flap.
Dry matter Calculation Formula (%)
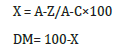
Where A= weighing bottle and sample weight before drying (g);
Z= weighing bottle and sample weight after drying (105 °C) (g)
C= Empty weighing bottle weight
The results are expressed inform of percentage (%).
Productivity and biochemical parameters
The performance of each inflorescence was evaluated based on parameters such as inflorescence count, length, number of fruits per inflorescence, average fruit mass per inflorescence, fruit dry matter per inflorescence, fruit height per inflorescence, and fruit diameter per inflorescence. Data were compiled from each row and presented in Table 2. The length and type of inflorescences were recorded for the first six inflorescences of each plant, including observations on uniparous cyme, biparous cyme, and multiparous cyme. Fruitkeeping quality was assessed by enclosing two representative fruits from each inflorescence in polythene bags and monitoring the number of days they remained viable at room temperature. Fruit size and mass per inflorescence were determined by individually weighing fruits and summing their mass, while fruit height and diameter were measured for five randomly selected fruits from the initial twelve inflorescences. Fruit firmness was assessed using a penetrometer on five fruits, and fruit freshness and dry matter content were determined by weighing samples before and after drying. Nitrate and sugar contents were measured using a portable tester and refractometer, respectively, while titratable acidity was determined by titrating samples with 0.1N NaOH until reaching a pH of 8.
Result
Figure 2 illustrates the monthly averages for various greenhouse microclimate parameters, including carbon dioxide levels, sunlight exposure, and air temperatures. These microclimatic conditions play a crucial role in influencing plant development, affecting processes such as photosynthesis and overall plant health. During the growth phase (May and June), the greenhouse typically experiences average air temperatures between 25.34 °C and 20.37 °C. Throughout the fruit-bearing and fruit-ripening stages (July and August), the average temperature ranged from 20.26 °C minimum to 23 °C maximum. In the pivotal growth phases of May and June, the greenhouse experiences a dynamic interplay of light intensity, with peaks and troughs ranging from 356.84 to 398.09 (W/m²), along with average CO2 concentrations spanning from 459.48 (ppm) to 495.84 (ppm). As plants reach the stages of fruit-bearing and ripening throughout June and July, a different atmospheric symphony unfolds. Here, light intensities and CO2 levels fluctuate between 446.06 to 479.27 (W/m²), painting a vivid tableau for the cherry tomatoes to flourish. The establishment and maintenance of an environment distinguished by optimum temperature, light, and carbon dioxide (CO2) levels are paramount for the vigorous horticultural advancement of cherry tomatoes. These conditions underpin crucial physiological processes, guaranteeing proficient photosynthesis, nutrient absorption, and comprehensive plant maturation [25]. Controlled settings, such as greenhouses or indoor cultivation facilities, afford cultivators the opportunity to meticulously calibrate these variables, thereby fostering enhanced yields and superior quality in cherry tomatoes.
Figure 2:Recorded average values of (a) Temperature °C, (b) Carbon Dioxide (ppm) and (c) Light intensity (W/m²) in the greenhouse growing compartment.

Growth patterns and vegetative characteristics in response to prolonged greenhouse conditions
Data regarding the growth response of plants to available greenhouse conditions is summarized in Table... The statistical interpretation of the revealed data showed a significant interaction and influence of greenhouse climatic conditions on the growth attributes of cherry tomatoes on different observation dates. In each row, every plant showed an immensely significant interaction (P<0.001) between overall growth parameters and observation dates (Table 1). A negative linear relation has been observed between total plant height and the combined effect of temperature, CO2, and light intensity treatments (“r” = -0.536, -0.686, and -0.57, respectively). but the height of plants significantly (P<0.001) differed on different observation dates. On the day of final observation, the plants’ maximum height was calculated at 308.64 centimeters. The strength of the relationship between the combined effect of temperature and CO2 and the weekly change in total height growth rate was observed to be positive (“r” = 0.32, 0.219, respectively). A negative linear relation between the light variations and the change in plant height rate has been observed (“r” = -0.103). The growth rate of plants was observed at the highest rate at the vegetative stage of growth during the months of May and June. The available greenhouse conditions during this period have been declared to be the optimum value for the plant’s vegetative growth stage. Stem diameter and leaf area of each plant significantly varied among observation dates (P<0.001) and also appeared in negative relation to the available greenhouse microclimate (Table 1). The leaf area serves as a powerful gauge of a plant’s vitality and yield potential. Our study unveils a compelling correlation between leaf area and productivity, showcasing that plants boasting larger leaf areas tend to bear more fruit. A direct relationship was found between the leaf area and inflorescence yield performance. The higher the leaf area, the higher the number of fruits produced by associated inflorescence. Leaf number 5 appeared with the highest leaf area, followed by 4 and 8. A significant difference was found among the dry matter contents of leaves. Leaf dry matter contents also appeared in direct relation to leaf area. Leaf count per plant varied significantly across observation dates, with an average emergence of 3 to 5 leaves per week. Despite fluctuations, the cumulative leaf count steadily increased throughout the observation period, persisting until the last day of observation.
Table 1:Growth attributes (mean values ± SD) in response to greenhouse conditions in summer-grown cherry tomatoes. Note: * p < .05, ** p < .01, *** p < .001.

Assessment of productivity and biochemical attributes: Correlation analysis
Productivity and biochemical attributes were assessed based on the data collected from the first six inflorescences (Table 2). illustrates the yield performance of the first six inflorescences in response to available greenhouse microclimatic conditions. According to the statistical interpretation of the collected data, a significant relationship was found (P<0.001) among the inflorescences in view of their length. Inflorescence number 5th appeared with the highest index, followed by inflorescence numbers 4th and 6th. Total fruit mass and number of fruits per inflorescence also significantly (P = 0.005) differed among the inflorescences (Figure 3). Inflorescence number 5 produced a larger fruit yield, followed by inflorescence numbers 4 and 6. The present study also observed a strong relationship between the inflorescence length, number of fruits (P<0.003), and total mass (g) produced by each inflorescence. The higher the inflorescence length, the higher the number of fruits and ultimately the total mass (g). A non-significant relation (P = 0.532) was observed among the inflorescences in view of average fruit mass. Inflorescence number 5 had the highest average fruit mass index, followed by inflorescence numbers 3rd and 2nd.
Table 2:Productivity attributes (mean values ± SD) in response to greenhouse conditions in summer-grown cherry
tomatoes.
Note: * p < .05, ** p < .01, *** p < .001

Figure 3:Productivity performance of first six inflorescence(a) Inflorescence length (b) Total mass inflorescence-1 (c) number of fruits inflorescence-1 (d) Average fruit mass inflorescence-1 (e)Fruit firmness inflorescence-1.

Fruit-keeping days were also significantly varied among the inflorescences. The fruit-keeping quality of tomatoes is directly linked to their economic importance. Fruit-keeping attributes followed the same order as average fruit mass, showing their direct association. A non-significant (P = 0.312) relation found between the inflorescence fruit keeping index. Inflorescence with a high average fruit mass survived more days at room temperature.
Firmness quality also significantly (P = 0.014) varied among the inflorescences. The first three inflorescences appeared with a maximum high index of fruit firmness. Inflorescence number 2 appeared with the highest fruit firmness, followed by inflorescence numbers 3rd and 1st. Fruit at the start of each inflorescence appeared with high mass and size. Fruits with high mass showed a high fruit firmness index.
A non-significant (P = 0.225) relationship has been observed among the inflorescences in view of their nitrate contents (Figure 4). The nitrate contents have been observed to be high in inflorescence numbers, followed by inflorescence numbers 5 and 6. Assessment of the proper ratio of nitrate and nitrite concentrations in tomatoes is very necessary to address concerns regarding the nutritional profile of tomatoes. The sugar contents were also non-significantly (P = 0.081) varied among the inflorescences. Inflorescence number 1 appeared with the highest Brix index, followed by 2 and 10.
Figure 4:Biochemical performance of first six inflorescence (a)Nitrate concentration inflorescence-1 (b) Brix percentage inflorescence-1.

A co-relation analysis declared a direct relationship between yields attributable to available greenhouse temperatures (Figure 4). Available temperature ranges (23.16 °C and 20.26 °C) and light and CO2 (468.46 °C and 356.02 °C W/m2) and (479.27ppm and 446.06ppm a) appeared with maximum productivity influence during the months of July and August. A strong negative linear relation between temperature and inflorescence total length has been observed (Table 2 & Figure 4). An increase in temperature above the optimum threshold reduced the growth of inflorescence, which ultimately led to low productivity. Applied climatic temperature ranges negatively affect the total number of fruits, total mass, and nitrate concentration per inflorescence. Average fruit mass, fruit firmness, Brix, and fruit dryness showed strong positive relationships with greenhouse conditions.
Discussion
The growth attributes and developmental processes of tomato plants have been found to have a significant relationship with the greenhouse climate. Differential responses of plant growth attributes on different observation dates can be linked to the influence of temperature on plant processes, including photosynthesis, transpiration, and respiration. In a scenario of escalating climate change, the probability of air temperatures surpassing the ideal range for numerous species intensifies, potentially disrupting their habitats and survival patterns. Different plant species have different temperature requirements for optimal growth [32]. Within their optimal temperature range, plants can efficiently carry out photosynthesis, respiration, and other metabolic processes necessary for growth. Temperatures outside this range can impede plant growth rate, total number of leaves, and stem diameter. Optimal temperatures allow for maximum photosynthetic activity, promoting more robust growth [33], Consistent with the present findings, a study reported that under low night temperatures, high intensity of light substantially enhanced the growth of plants and led to an increase in various parameters such as plant height, stem diameter, leaf area, vigor index, and the overall dry weight of cherry-tomato seedlings [34]. Elevated concentrations of nighttime temperature prominently reduce the plant’s yield. This decline is often associated with the adverse impact of respiration on the accumulation of carbohydrates, further exacerbating the challenge of maximizing crop productivity [35]. In the present study, we observed a positive correlation between the average temperature range of -25.34 and 20.26 and the change in plant growth rate. Similar to it, a study established a linear relationship between suboptimal temperature ranges and lower growth rates following the production of thicker leaves, which hinder the interception of light [36].
According to [37] elevated CO2 levels can enhance plant growth and biomass by improving photosynthetic rates in leaves, increasing water use efficiency, and reducing transpiration. A notable difference in plant height has been reported, with elevated carbon EC700 reaching 152.20cm compared to 138.13cm in the open field [38]. This variation could be attributed to a higher rate of net photosynthesis in an elevated CO2 environment. The tomato plant, classified as a C3 plant, experiences advantages from high levels of carbon dioxide (CO2) in its environment [39]. Elevated CO2 concentrations enhance the rate of photosynthesis because the increased CO2 levels create a more favorable concentration gradient from the air to the leaf, leading to more efficient photosynthesis. heightened CO2 levels decrease photorespiration and result in reduced expression of Rubisco, a key enzyme in photosynthesis [40]. Light also plays a pivotal role in shaping the growth and progression of plants, serving as the primary driver for photosynthesis and an influential external cue. Its impact extends across multiple dimensions of plant life, influencing not only their physical characteristics but also the intricacies of physiological functions, yield production, and the ultimate quality of the harvest. Photoperiod refers to the amount of time a plant is exposed to light [41-43]. Photoperiod controls flowering in many plants (Figure 1). Scientists used to think that the length of the light period triggered flowering and other responses within plants.
Different extreme ranges of greenhouse climate have profound effects on plant productivity and yield. A study established a linear positive correlation between the extreme temperature level and a higher number of fruits [44]. The present study found a direct relation between the yield and quality of summer-grown tomato fruits in the available temperature ranges of 23.16 °C and 20.26 °C. Consistent with the present findings, [45], reported a positive linear co-relation between the temperature range of -25.9 °C and 22.2 °C and the yield attributes of summer-grown tomatoes. Temperature plays a crucial role in shaping the quality of fruits. Daytime temperatures that are too low often produce poor growth by slowing down photosynthesis, i.e., the number of fruits. Its impact on metabolism directly influences cellular structure and various factors determining fruit quality, including color, texture, size, and sensory attributes. Lower temperatures increase the time required for ripening and, therefore, increase the size of fruits [46]. The interplay of temperature and daylight duration can influence the flowering process. When temperatures rise alongside longer days, it prompts cool-season plants like spinach to initiate flowering. Conversely, when temperatures drop too low, it can hinder the fruit setting process in warm season crops like tomatoes. Only a handful of researchers have delved into exploring how temperature and cultivar dynamics impact yield. Different tomato cultivars exhibit variations during vegetative growth; the nexus between temperature and cultivar appears negligible. At optimal temperature, the yield varies across tomato types such as “cherry,” “round,” and “beefsteak,” primarily influenced by distinct assimilate partitioning mechanisms. In the present study, we found a significant relationship between the leaf area below the inflorescence and the productivity performance of that inflorescence. Thus, the production of leaves serves as an important phenomenon during fruit development (Figure 5). A study investigating how different environmental factors (temperature, solar radiation, and vaporpressure deficit) affect the nutritional quality and flavor of cherry tomatoes reported a reduction in lycopene and essential elements and an increase in the sweeter-milder flavor profile with increased sugars and decreased organic acid content [47]. Rising temperatures and shifting precipitation trends could lead to heightened depletion of soil minerals, primarily through leaching and erosion processes. To mitigate such losses and enhance plant production, exploring soilless systems and understanding greenhouse cultivation could present a promising alternative. The escalation in CO2 levels, coupled with the surging presence of various other greenhouse gases, has resulted in a notable uptick of 0.8 °C in average annual global temperatures by 2017 [48]. This trend underscores the pressing need for heightened awareness and decisive action to mitigate further climate disruptions and their damage to the agriculture sector [49].
Figure 5:The co-relation demonstration of different yield attributes with Temperature (a) Inflorescence length (b) Total fruits inflorescence-1 (c) Total mass inflorescence-1 (d) Dry matter contents inflorescence-1 (e) Nitrate concentration inflorescence-1(f) Brix percentage inflorescence-1.

Conclusion
The study investigated the influence of climatic factors in greenhouses on the growth, yield, and biochemical attributes of summer-grown tomatoes in soilless systems on different observation dates. A Significant interaction has been found between certain growth attributes and observation dates. The results reveal both positive and negative linear correlations between greenhouse factors and productivity attributes. Based on Positive correlations between available climatic conditions, such as temperature, CO2 and light intensity, optimum climatic conditions have been proposed. This research offers a way out for establishing optimal conditions for future tomato cultivation and for identifying highly susceptible varieties under extreme conditions, aiding in more resilient agricultural practices. By integrating these strategies, growers can enhance the resilience and adaptability of tomato production systems, ensuring consistent yields and nutritional quality regardless of fluctuating climatic conditions.
Acknowledgment
The authors acknowledge prof. dr. Adrian Asanica, Dean Faculty of Horticulture, prof. dr. Florin Stanica, head of the department of pomology lab at the University of Agri-culture and Veterinary Medicine Bucharest, for technical support. We are also grateful to Petre Andrei-Catalin, Frincu Mihai, Mis, Aurora, Mis, Ana Butcaru for laboratory assistance, and Mis, Simona, Mis, Gina, and Mr. Bogdan for greenhouse management. The research leading to these results has received funding from the NO Grants 2014-2021, under project contract no. 40/2021.
References
- Xu SY, Weng JK (2020) Climate change shapes the future evolution of plant metabolism. Advanced Genetics 1(1): e10022.
- Arshad A (2021) A growth and biochemistry of ten high yielding genotypes of Pakistani rice (Oryza sativa) at maturity under elevated tropospheric ozone. Heliyon 7(10): e08198.
- Rather Amud, Hajam MA, Bhat MSA, Malik MI (2023) Horticulture: Principles and practices. Academic Guru Publishing House, Bhopal, India, pp. 217.
- Debjit B, Kumar KS, Paswan S, Srivastava S, Shweta S (2012) Tomato-a natural medicine and its health benefits. Journal of Pharmacognosy and Phytochemistry 1(1): 33-43.
- Costa JM, Heuvelink EP (2018) The global tomato industry. Tomatoes 27: 1-26.
- Elbadrawy E, Sello A (2016) Evaluation of nutritional value and antioxidant activity of tomato peel ex-tracts. Arabian Journal of Chemistry 9(2): S1010-S1018.
- Chaudhary P, Sharma A, Singh B, Nagpal AK (2018) Bioactivities of phytochemicals present in tomato. Journal of Food Science and technology 55(8): 2833-2849.
- Ramos-Bueno RP, Romero-Gonzalez R, Gonzalez-Fernandes MJ, Guil-Guerrero JL (2017) Phytochemical composition and in vitro anti-tumour activities of selected tomato varieties. J Sci Food Agric 97(2): 488-496.
- Dawid J (2016) The role of tomato products for human health (Solanum lycopersicum)-A review. Journal of Health, Medicine and Nursing 33: 66-74.
- Gatahi DM (2020) Challenges and opportunities in tomato production chain and sustainable standards. International Journal of Horticultural Science and Technology 7(3): 235-262.
- Costa JM, Heuvelink E (2007) Today's worldwide tomato production. Fruit & Veg Tech, pp. 14-16.
- Agius C, von Tucher S, Rozhon W (2022) The effect of salinity on fruit quality and yield of cherry tomatoes. Horticulturae 8(1): 59.
- Arshad A, Jerca IO, Chan S, Cîmpeanu SM, Teodorescu RI, et al. (2023) Study regarding the influence of some climatic parameters from the greenhouse on the tomato production and fruits quality. Scientific Papers. Series B, Horticulture 67(2):
- Jones JB (2007) Tomato plant culture: in the field, greenhouse, and home garden. CRC press, Florida, USA.
- Sato S, Peet M, Thomas J (2000) Physiological factors limit fruit set of tomato (Lycopersicon esculentum) under chronic, mild heat stress. Plant Cell Environ 23(7): 719-726.
- Charles WB, Harris R (1972) Tomato fruit-set at high and low temperatures. Can J Plant Sci 52(4): 497-506.
- Beckles DM (2012) Factors affecting the postharvest soluble solids and sugar content of tomato (Solanum lycopersicum) fruit. Postharvest biology and technology 63(1): 129-140.
- Jones RB (2007) Effects of postharvest handling conditions and cooking on anthocyanin, lycopene, and glucosinolate content and bioavailability in fruits and vegetables. New Zealand Journal of Crop and Horticultural Science 35(2): 219-227.
- Jerca IO, Cîmpeanu SM, Teodorescu RI, Drăghici EM, Nițu OA, et al. (2024) A comprehensive assessment of the Morphological development of inflorescence, yield potential, and growth attributes of summer-grown, greenhouse cherry tomatoes. Agronomy 14(3): 556.
- Jerca IO, Cîmpeanu SM, Teodorescu RI, Țiu,J, Postamentel M, et al. (2023) The effect of improving the climatic conditions in the greenhouse on the cheramy tomato hybrid grown in greenhouse conditions. Scientific Papers. Series B. Horticulture 67(2):
- Drake JE, Gallet‐Budynek A, Hofmockel KS, Bernhardt ES, Billings SA, et al. (2011) Increases in the flux of carbon belowground stimulate nitrogen uptake and sustain the long‐term enhancement of forest productivity under elevated CO2. Ecology Letters 14(4): 349-357.
- Bisbis MB, Gruda N, Blanke M (2018) Potential impacts of climate change on vegetable production and product quality- A Review. J Clean Prod 170: 1602-1620.
- Long SP, Ainsworth EA, Rogers A, Ort DR (2004) Rising atmospheric carbon dioxide: plants FACE the future. Annu Rev Plant Biol 55: 591-628.
- Emana B, Afari-Sefa V, Nenguwo N, Ayana A, Kebede D, et al. (2017) Characterization of pre-and postharvest losses of tomato supply chain in Ethiopia. Agriculture & Food Security 6: 1-11.
- Hewett EW (2006) An overview of preharvest factors influencing postharvest quality of horticultural products. International Journal of Postharvest Technology and Innovation 1(1): 4-15.
- FAO (1989) Prevention of food losses: fruit, vegetable and root crops: A training manual. Food and Agriculture Organization of the United Nations, Rome, Italy.
- Kader AA (2005) Increasing food availability by reducing postharvest losses of fresh produce. Acta Hort 682: 2169-2176.
- Alfeo V, Planeta D, Velotto S, Palmeri R, Todaro A (2021) Cherry tomato drying: Sun versus convective oven. Horticulturae 7(3): 40.
- Harel D, Fadida H, Gantz S, Shilo K, Yasuor H (2013) Evaluation of low pressure fogging system for improving crop yield of tomato (Lycopersicon esculentum): Grown under heat stress conditions. Agronomy 3(2): 497-507.
- Carmassi G, Incrocci L, Incrocci G, Pardossi A (2007) Non-destructive estimation of leaf area in Solanum lycopersicum and gerbera (Gerbera jamesonii H. Bolus). Agr Med 137: 172-176.
- Alsadon AA, Al-Helal IM, Ibrahim AA, Shady, MR, Al-Selwey WA (2018) Growth analysis of tomato plants in controlled greenhouses. In XXX International Horticultural Congress IHC2018: III International Symposium on Innovation and New Technologies in Protected 1271: 177-184.
- Hatfield JL, Prueger JH (2015) Temperature extremes: Effect on plant growth and development. Weather and Climate extremes 10: 4-10.
- Went FW (1944) Plant growth under controlled conditions. II. Thermoperiodicity in growth and fruiting of the tomato. American Journal of Botany 31(3): 135-150.
- Song J, Chen Z, Zhang A, Wang M, Jahan MS, et al. (2022) The positive effects of increased light intensity on growth and photosynthetic performance of tomato seedlings in relation to night temperature level. Agronomy 12(2): 343.
- Loka D, Oosterhuis D (2010) Effect of high night temperatures on cotton respiration, ATP levels and carbohydrate content. Environ Exp Bot 68(3): 258-263.
- De Koning ANM (2000) The effect of temperature, fruit load and salinity on development rate of tomato fruit. Acta Horticulturae 519: 85-93.
- Jones HG (2013) Plants and microclimate: A quantitative approach to environmental plant physiology. (3rd edn), Cambridge University Press; Cambridge, UK.
- Rangaswamy TC, Sridhara S, Ramesh N, Gopakkali P, El-Ansary DO, et al. (2021) Assessing the impact of higher levels of CO2 and temperature and their interactions on tomato (Solanum lycopersicum). Plants (Basel)10(2): 256.
- Wittwer SH, Strain BR (1985) Carbon dioxide levels in the biosphere: effects on plant productivity. Critical Reviews in Plant Sciences 2(3): 171-198.
- Lenka NK, Lenka S, Thakur JK, Elanchezhian R, Aher SB, et al. (2017) Interactive effect of elevated carbon dioxide and elevated temperature on growth and yield of soybean. Curr Sci 113(12): 2305-2310.
- He D, Yan Z, Sun X, Yang P (2020) Leaf development and energy yield of hydroponic sweetpotato seedlings using single node cutting as influenced by light intensity and led spectrum. J Plant Physiol 254: 153274.
- Abidi F, Girault T, Douillet O, Guillemain G, Sintes G, et al. (2013) Blue light effects on rose photosynthesis and photomorphogenesis. Plant Biol 15(1): 67-74.
- Ji F, Wei S, Liu N, Xu L, Yang P (2020) Growth of cucumber seedlings in different varieties as affected by light environment. Int J Agric Biol Eng 13(5): 73-78.
- Adams SR, Cockshull KE, Cave CRJ (2001) Effect of temperature on the growth and development of tomato fruits. Annals of Botany 88(5): 869-877.
- Mulholland BJ, Edmondson RN, Fussell M, Basham J, Ho LC (2003) Effects of high temperature on tomato summer fruit quality. The Journal of Horticultural Science and Biotechnology 78(3): 365-374.
- Dorais M, Papadopoulos AP, Gosselin A (2001) Greenhouse tomato fruit quality. Horticultural Reviews 26: 239319.
- Rosales MA, Cervilla LM, Sánchez‐Rodríguez E, Rubio‐Wilhelmi MDM, Blasco B, et al. (2011) The effect of environmental conditions on nutritional quality of cherry tomato fruits: evaluation of two experimental Mediterranean greenhouses. Journal of the Science of Food and Agriculture 91(1): 152-162.
- Hansen J, Ruedy R, Sato M, Lo K (2010) Global surface temperature change. Reviews of Geophysics 48(4): 1-29.
- Miraglia M, Marvin HJP, Kleter GA, Battilani P, Brera C, et al. (2009) Climate change and food safety: an emerging issue with special focus on Europe. Food and Chemical Toxicology 47(5): 1009-1021.
© 2024 Elena Maria Draghici. This is an open access article distributed under the terms of the Creative Commons Attribution License , which permits unrestricted use, distribution, and build upon your work non-commercially.
 a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in
a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in 







.jpg)






























 Editorial Board Registrations
Editorial Board Registrations Submit your Article
Submit your Article Refer a Friend
Refer a Friend Advertise With Us
Advertise With Us
.jpg)






.jpg)














.bmp)
.jpg)
.png)
.jpg)










.jpg)






.png)

.png)



.png)






