- Submissions

Full Text
Modern Concepts & Developments in Agronomy
Photosynthetic Characteristics of Younger Leaves (Emerging Leaves) and Older Leaves (Mature Leaves) of Various Field-Grown J. Curcas Accessions
Hilary Shoniwa* Baleseng Moseki and John Choinski
Department of Biological Sciences, Gaborone Botswana
*Corresponding author:Hilary Shoniwa, Department of Biological Sciences, University of Botswana, Private Bag UB00704, Gaborone Botswana
Submission: February 16, 2024;Published: March 13, 2024

ISSN 2637-7659Volume14 Issue 1
Abstract
Jatropha curcas is an oilseed-bearing shrub with high potential for biodiesel production in arid regions, where it is also subject to abiotic stress such as high light and high temperatures. As a plant, being sessile, its survival therefore depends on the development and efficient activation of resistance responses to these stressors. The photosynthetic process is vital for plant growth and survival and the leaves are the major photosynthetic organs. The life cycle of a leaf is made of different stages viz., the emerging leaf, the mature leaf and the last stage, the senescing leaf. The aim of the study was two-fold:
(i) assessing the degree of photoinhibition from the emerging leaf stage (young leaf) through their development to mature leaf stage (older leaves) and
(ii) investigating their photoprotective mechanism(s).
The plants were raised in a field located at Sebele, Botswana The field was established in 2011 from seedlings planted from the seeds of various J. curcas accessions collected from different areas of Botswana (Tsamaya from the north, Tabala from the central region and Tlokweng from the Southeast region). The fourth accession was obtained from Ghana. Gas exchange, chlorophyll fluorescence, photosynthetic pigments and antioxidants were studied. The younger leaves of all accessions (7 days old) exhibited lower photosynthetic rates and lower photosynthetic pigments than the older leaves (35 days old). Ghana and Tlokweng accessions appeared to exhibit higher photosynthetic rates than the Tsamaya and Tabala accessions in all leafs developmental stages. All accessions generally displayed lower photosynthetic rates and dark-adapted Fv/Fm ratios at 13:00hrs than at 07:00hrs. The decline in dark-adapted Fv/Fm ratio and photosynthetic rate exhibited by the accessions at midday can be attributed to higher midday photosynthetic active radiation and temperature. Since the accession exhibited full recovery of their dark-adapted Fv/Fm ratio and photosynthetic rate by late afternoon, the decrease in these two parameters can be associated with high carotenoid levels. Ghana and Tlokweng accessions exhibited higher levels of antioxidants (SOD and CAT) and carotenoids than Tabala and Tsamaya accessions which protect them from effects of high photosynthetic radiation and high temperatures. It is therefore, concluded that the Tlokweng and Ghanaian accessions can be recommended for growing in Botswana (semi-arid country), as valuable feedstocks to produce biodiesel, which is environmentally friendly
Keywords:
Jatropha curcas; Dark-adapted Fv/Fm ratio; Antioxidants; Photosynthetic rates; Photosynthetic pigmentsIntroduction
Jatropha curcas is a perennial shrub belonging to the Family Euphorbiaceae and it can grow up to 8 m under favourable conditions [1]. Jatropha curcas originated in Meso-America but has now spread around the world, including semi-arid regions in Africa [2]. The plant species is of great socio-economic importance and environmental importance [3,4]. It is also used in the pharmaceutical industry and cosmetic industry. In addition, it has gained in importance because its seeds contain oil that can be used in biodiesel production [5], Keira and Atta, 2009).
Emerging leaves are at the early stage of the life cycle of leaves. Emerging leaves, therefore, grow to become mature leaves and these will become senescent and die [6]. Massaoudou et al. [7] from their studies of J curcas in the Sahel of Niger describe three phases of leaf growth. The early phase, starting two days after identification of the bud which grows slowly up to six days; the second phase characterised by strong growth from about seventh day to the twelfth day; the third phase from the thirteenth day to around the 35th day when growth plateaus. Early in leaf development, both photosynthetic rate and dark-adapted Fv/Fm ratio, photosynthetic pigments (chlorophyll and carotenoids) and stomatal conductance gradually increase. Low CO2 absorption rates and stomatal closure coincide during the early stages of leaf growth because photosynthetic machinery is still developing [8,9]. While the developing young leaves may be subjected to significant photoinhibition due to inefficient photosynthetic machinery, low levels of chlorophyll limit light absorption and hence possible photodamage [6].
On exposure to high irradiance, only a portion of absorbed energy is used in CO2 assimilation. The rest is dissipated to avoid photodamage to the emerging leaves Jiang et al. [10,11] and Juvany et al. [6] report that many researchers have established that plants have evolved several systems of reducing photodamage. The carotenoids dissipate excess energy through the xanthophyll cycle [12]. Asada [13] and Jiang et al. [10] reported that excess light can activate the production of Reactive Oxygen Species (ROS) through the electron transport chain, and this can lead to the production of superoxide anion (O¯₂). Jiang et al. [10] noted that antioxidant enzymes, viz., Superoxide Dismutase (SOD) and Catalase (CAT) are active at the early stages of the leaf expansion when their levels were highest and therefore, possibly playing the role of scavenging Reactive Oxygen Species (ROS).
The aim of the study was two-fold:
(i) assessing the degree of photoinhibition from the emerging
leaf stage (young leaf) through their development to mature
leaf stage (older leaves) and
(ii) investigating their photoprotective mechanism(s).
This was achieved by assessment of changes in the photosynthetic rate and in the maximum photochemical efficiency of PSII (estimated from dark-adapted Fv/Fm ratio) and investigating their photo-protective mechanism(s) through measurements of carotenoids and antioxidant (SOD and CAT) levels in field-grown J. curcus accessions.
Materials and Methods
Study site
The growth measurements were carried out at an agricultural field located in the Department of Agricultural Research, Sebele, Botswana [14]. It is a semi-arid area with a wide range of diurnal temperatures throughout the year. The average precipitation in this area is below 490mm annually [14]. Precipitation occurring from October to March accounts for almost 100% of the annual rainfall [14]. Summer temperatures range from 15 °C in the morning to over 40 °C at midday and winter temperatures range from 3 °C early morning to 25 °C in the afternoons [14]. The soil is reddish brown and is of the Rendzic Leptosol type. They are poor soils with high aluminium and iron content consisting of silt and clay [14].
The field was established in 2011 from seedlings planted from the seed of various J. curcas accessions collected from different areas of Botswana (Tsamaya from the north, Tabala from the central region and Tlokweng from the Southeast region). One of the accessions was obtained from Ghana. The experiment was conducted in October to May in 2015, 2016 and 2017. The plants were grown in an area of 0.5ha with a plant spacing of 2m x 2m between the plants.
Experimental design and treatments
The field experiment was laid out in a randomized block design with five replications. The treatments were four J. curcas accessions namely, Tsamaya, Thabala, Tlokweng, and Ghana. These accessions were randomly selected from several parts of the country and the accession from Ghana was regarded as the control, since it has been widely studied.
Photosynthetic rate measurements
Photosynthetic rate (μmol CO2 m¯²sˉ¹), measurements were taken using a portable photosynthesis system (LICOR 6400XT equipped with a LED 2x3cm leaf chamber, LICOR USA). Diurnal measurements were first carried out on the youngest stage (day 7) and continued to be taken every seven days up to the mature stage at day 35. Each day the measurements were taken twice at 0700h and at midday.
Chlorophyll fluorescence measurements
The measurements of the maximum photochemical efficiency of PSII (estimated from dark-adapted Fv/Fm ratio were determined after the leaves were dark-adapted for 30 min [15], using a fluorimeter (Hansatech Instruments Ltd, Norfolk, UK). The darkadapted Fv/Fm ratio measurements were taken three times a day at 0700h 1200h and 1800h. These dark-adapted Fv/Fm ratio measurements were first carried out on leaves at the youngest stage (day 7) and continued to be taken at day 14, day 21, day 28 and day 35, the mature stage.
Photosynthetic pigments quantification
Chlorophyll and carotenoid contents were determined according to Lichtenthaler et al. [16]. 0.2g of leaf tissue was macerated using a mortar and pestle. The macerated tissue was placed in 10 ml of 80% acetone for total pigment extraction. The crude extract was centrifuged at 3000rpm for 5 minutes and the pellet was discarded. The supernatant was used to determine absorbance by a UV mini 1240UV-VIS spectrophotometer (Shimadzu, Tokyo Japan) at 470, 649, 530, and 470 nanometers. The chlorophylls and carotenoids were calculated according to Lichtenthaler et al. [16] and Sims and Gamon (2002) using the following equations:
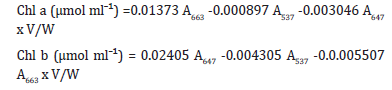
Carotenoids (μmol mlˉ¹) = [A470-(17.1x (chl a+chl b)-9.479x anthocyanin)]/119.26 xV/W Where, A646 =absorbance at a wavelength of 646nm, A663 = absorbance at a wavelength of 663nm A470 = absorbance at a wavelength of 470nm, A537= absorbance at a wavelength of 537nm A530 = absorbance at a wavelength of 530nm, V=volume of sample (ml 80% acetone) & W=weight of sample in g. Mean chlorophyll and carotenoid content were expressed in μg gfwt¯¹ where fwt denotes fresh weight
Determination of superoxide dismutase (SOD)
SOD was measured/determined as previously described Giannopolitis et al. [17]. 0.2g of leaf tissue were homogenized using chilled glass mortar and pestle in a medium consisting of 50mM phosphate buffer (pH 7.8) and 100mg of polyvinyl polypyrolidone as phenolic binder. The homogenate was centrifuged at 16000x g for 15 minutes in a refrigerated centrifuge at 4 °C. The supernatant was collected for the assay. Different sets of the assay system were prepared separately as follows: one for the assay, one for the dark control and one for the light control. The reaction mixture consisted of 0.1ml of 1.5M sodium carbonate, 0.3ml of 0.13M methionine, and 0.3ml of 10μM EDTA, 0.3 ml of 13μM riboflavin, and 0.3ml of 0.63mM nitroblue tetrazolium. The nitroblue tetrazolium was withheld in the light control system. To the reaction system, 0.1ml of enzyme extract was added to the blank and to the lightcontrol system. The reaction mixture was made up to 3.0ml using 50mM phosphate buffer (pH 7.8). The tubes with dark control samples (blank: all reagents and sample added) were kept in a dark chamber and the tubes of the light control (all reagents except nitroblue tetrazolium with sample were added just before reading absorbance) and the assay systems (all reagents and sample) were kept under a fluorescent lamp. After 30 minutes of incubation the optical densities of the solutions of the assay, the dark control and the light control systems were measured at 560nm using a spectrophotometer (UV mini -1240 UV-VIS, Shimadzu Tokyo Japan). One unit of SOD was defined as enzyme activity that inhibited the reduction of nitro-blue tetrazolium to blue formazin by 50% or the amount of enzyme which reduced the absorbance reading to 50% in comparison to tubes lacking enzyme.
SOD was expressed in Units per milligram (Units/mg) SOD Activity=(A0-A1)/A0 ÷50%×(System Volume)/(Sample Volume)
A0 is the absorbance in the absence of enzyme extract. A1 is the absorbance in the presence of enzyme extract. The enzymatic activity that causes 50% inhibition in the system is defined as one unit (Zhang et al., 2016).
Determination of catalase activity
Catalase activity was measured according to Kar & Mishra [18]. Enzyme extract was prepared by grinding 0.2g leaf tissue in a chilled mortar and pestle by adding 4ml 50Mm phosphate buffer pH 7 mixed with 100mg polyvinyl polypyrolidone as phenolic binder and centrifuged for 15 minutes at 4000rpm (4 °C). The assay consisted of 1.0ml 3% hydrogen peroxide. The phosphate buffer and enzyme extract were pipetted out and mixed well in a test tube. To this 3% hydrogen peroxide was added to initiate enzyme activity. Immediately after the addition of hydrogen peroxide, enzyme activity was measured at 240nm for 180 seconds at 15-second intervals using Spectrophotometer, and 1ml 50mM phosphate buffer (pH 7) was used as blank.
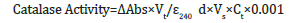
where, ΔAbs is the difference between the initial and final absorbance, Vt is total volume of reaction, Ɛ240 is the molar extinction coefficient for H2O2 at OD240 (34.9 mol−1 cm−1), d is optical path length of cuvette (1cm), Vs is volume of sample, Ct is the total protein concentration in the sample and 0.001 is absorbance change caused by 1U of enzyme per min at 240nm OD.
Result
Photosynthetic rates of younger leaves (emerging leaves) and older leaves (mature leaves) of various J. curcas accessions
The younger leaves of all accessions (7 days old) exhibited lower photosynthetic rates than the older leaves (day 35) (Figure 1). The accessions generally displayed lower photosynthetic rates at (1300h) than at 0700h independent of leaf developmental stage. Generally, Ghana and Tlokweng accessions appeared to exhibit higher photosynthetic rates than the Tsamaya and Tabala accessions in all leaf developmental stages.
Figure 1:Diurnal photosynthetic rates of several J. curcas accessions grown under field conditions. Means followed by different letters are significant at P≤0.05 according to Fisher LSD. Bars represent standard error of the mean (SEM) (n=5).

Maximum photochemical efficiency of PSII, estimated from dark-adapted Fv/Fm ratios of younger leaves (emerging leaves) and older leaves (mature leaves) of various J. curcas accessions
All the accessions displayed lower dark adapted Fv/Fm ratios at midday (1300h) compared to the morning ratios (Figure 2). Generally, throughout the 35 days, the accessions differed significantly (P≤0.05) in their dark adapted Fv/Fm ratios. The Ghana and Tlokweng accessions largely exhibited higher dark-adapted Fv/Fm ratios at midday than the Tsamaya and Tabala accessions (Figure 2).
Figure 2:Maximum photochemical efficiency of PSII (estimated from Dark-adapted Fv/Fm ratios for J. curcas accessions. Means followed by different letters are significant at P≤0.05 according to Fisher LSD. Bars represent SEM (n=5).

Figure 3 shows that all the accessions at day 7-day leaf development exhibited lower dark-adapted Fv/Fm ratios at midday and by the late afternoon recovered beyond their initial dark adapted Fv/Fm ratio at 0700h. At each time during the day the darkadapted Fv/Fm ratio of the accessions differed significantly (P≤0.05) from each other. Throughout the day Ghana, accession displayed the lowest dark-adapted Fv/Fm ratio, compared to the other accession.
Figure 3:The maximum photochemical efficiency of PS II (estimated from dark-adapted Fv/Fm ratio) for young leaves of J. curcas accessions. Means followed by different letters are significant at P≤0.05 according to Fisher LSD. Bars represent SEM (n=5).

Photosynthetic pigments of J. curcas accessions of the younger leaf stage (emerging leaves) to the older leaf (mature leaves) stage.
All accessions appeared to exhibit lower chlorophyll content (Figure 4) and carotenoid content (Figure 5) at the younger leaf stage of development (7-day old). The Ghana accession exhibited slightly lower chlorophyll content during the younger stages of development compared to the other three accessions, but their carotenoids (Figure 4) were more or less the same. However, as the leaves developed the Ghana and Tlokweng accessions displayed the highest chlorophyll content (Figure 4) and carotenoid (Figure 5) compared to Tabala and Tsamaya accessions.
Figure 4:Chlorophyll levels of the leaves of J. curcas accessions in field conditions in the Southeast District of Botswana a semi-arid country. Means followed by different letters are significant at P≤0.05 according to Fisher LSD. Bars represent SEM n=5.

Figure 5:Carotenoid contents from the leaves of J. curcas accessions in field conditions in the Southeast District of Botswana a semi-arid country. Means followed by different letters are significant at P≤0.05 according to Fisher LSD. Bars represent SEM (n=5).

Diurnal Antioxidants contents (SOD and CAT) of J. curcas accessions from young (emerging) leaves to mature (older) leaves
All accessioned appeared to display highest SOD contents (Figure 6) and CAT contents (Figure 7) at the younger stage of development (7-day old) regardless of time of day. Both the SOD and CAT contents of the accessions were highest around noon, when the leaves experienced highest photosynthetic active radiation (PAR) (Figure 8), followed by morning and then late afternoon. Throughout the 35-day period, the SOD contents (Figure 6) and CAT contents (Figure 7) of the accessions differed significantly (P≤0.05). At almost each time of the day and each stage of development the Ghana and Tlokweng accessions, displayed slightly higher SOD levels than the Tsamaya and Tabala accessions (Figure 6 & 7). On the other hand, younger leaves exhibited the highest levels of CAT compared to the older leaves (Figure 7), especially at mid-day (noon). (Figure 7).
Figure 6:Carotenoid contents from the leaves of J. curcas accessions in field conditions in the Southeast District of Botswana a semi-arid country. Means followed by different letters are significant at P≤0.05 according to Fisher LSD. Bars represent SEM (n=5).

Figure 7:Carotenoid contents from the leaves of J. curcas accessions in field conditions in the Southeast District of Botswana a semi-arid country. Means followed by different letters are significant at P≤0.05 according to Fisher LSD. Bars represent SEM (n=5).

Photosynthetic active radiation (PAR) and Leaf temperature
Figure 8 shows the diurnal variation of the Photosynthetic Active Radiation (PAR) over three years, 2015, 2016 and 2017. The leaves of all accessions appear to experience higher temperatures at noon, compared to morning and late afternoon (Figure 8). In 2017, the leaves experienced the highest PAR levels at midday but 2015 and 2016 their PAR levels were not significantly different (P≥0.05) at this time. The PAR in 2015 and 2017 was not significantly different (P≥0.05) at 0700h. At 1800h, the PAR in 2015 was significantly different (P≤0.05) from that of 2017.
Figure 8:Diurnal variation of the photosynthetic active radiation in each of the three (2015, 2016 and 2017) years of the study of selected J. curcas accessions in field conditions in Southeast Botswana a semi-arid country. Bars followed by the same letters on the graph show that the photosynthetic active radiation of those accessions were not significantly different at P≥0, 05 according to Fisher LSD. Each accession represents the mean of five different plants.

Diurnal leaf temperatures for various J. curcas accessions for each season of the years covered in the study 2015 to 2017
Figure 9 shows diurnal leaf temperatures of the selected J. curcas accessions for each season of 2016. In each season the midday leaf temperatures were the highest and the morning leaf temperatures were the lowest. Comparing the seasons, the summer leaf temperatures were highest at midday followed by autumn. The Ghana and Tsamaya accessions exhibited the highest leaf temperatures in summer which did not differ significantly from each other. In the autumn the Tlokweng and Tabala accessions did not differ significantly from each other at midday but differed significantly from the Ghana and Tsamaya accessions.
Figure 9:Diurnal variation of the interaction effect of accessions and time of day and season on the leaf temperatures of selected J. curcas accessions grown under field conditions in the Southeast of Botswana, a semiarid country in 2016. Bars followed by the same letters on the graph show that the photosynthetic rates of those accessions were not significantly different at P≤0, 05 according to Fisher LSD.

Figure 10 shows diurnal photosynthetic rates of the selected J. curcas accessions for each season of 2016. In each season the midday photosynthetic rates were lower than the morning late afternoon photosynthetic rates. Comparing the seasons, the summer photosynthetic rates were higher at midday followed by spring then autumn. Generally, the Ghana and Tlokweng accessions exhibited the higher photosynthetic rates in the seasons of this (2016) than the Tsamaya and Tabala accessions.
Figure 10:Diurnal variation of the interaction effect of accessions and time of day and season on the photosynthetic rates of selected J. curcas accessions grown under field conditions in the southeast of Botswana, a semi-arid country in 2016. Bars followed by the same letters on the graph show that the photosynthetic rates of those accessions were not significantly different at P≤0, 05 according to Fisher LS.

Discussion
The younger leaves of all accessions (7 days old) exhibited lower photosynthetic rates than the older leaves (day 35) (Figure 1). The lower photosynthetic rate exhibited by the younger leaves can be attributed to their lower chlorophyll content (Figure 4), compared to that of older leaves. The lower chlorophyll content exhibited by the younger, presumably serves as a protective mechanism, by minimizing capture of excitation energy (PAR), thus protecting the younger leaves from photodamage. These results are consistent with those of Choinski and Wise (1999) and Greer and Halligan 2001, who pointed out that low carbon assimilation in young leaves (Figure 1) was due to photosynthetic apparatus which were still under construction. The gradual increase in photosynthetic rates simultaneous with leaf development also points to the progressive development of the photosynthetic machinery (Figure 1). While the developing young leaves may be subjected to significant photoinhibition due to inefficient photosynthetic machinery, their low levels of chlorophyll contents (Figure 4) [19] limit light absorption and hence ameliorated effects of possible photodamage [6].
Diurnal measurements of photosynthetic rates demonstrated that all the accessions generally displayed lower photosynthetic rates at 13:00hr than at 07:00hrs independent of leaf developmental stage. It should be noted that at 13:00hrs, the accessions experienced high Photosynthetic Active Radiation (PAR) (Figure 8). The lower photosynthetic rates exhibited by the accessions at noon (Figure 10), can therefore, be ascribed to their higher carotenoid contents (Figure 5). The increase in carotenoid contents, which by inference could imply an increase in xanthophyll cycle pool, which in turn dissipated excess excitation energy harmlessly as heat [19,10,12]. Ghana and Tlokweng accessions appeared to exhibit higher photosynthetic rates than the Tsamaya and Tabala accessions in all leafs developmental stages. This can be attributed to their higher carotenoid contents (Figure 5), higher SOD content (Figure 6) and higher CAT (Figure 7) compared to the other accessions (Tabala and Tsamaya).
All the accessions at day 7 of leaf development exhibited the lowest dark-adapted Fv/Fm ratios at midday and by the late afternoon had recovered beyond their initial Fv/Fm ratio at 0700h (Figure 2). The low midday dark-adapted Fv/Fm ratio of the youngest leaves can be attributed to the fact that photosynthetic machinery was still developing (Choinski and Wise (1999). The decline in dark-adapted Fv/Fm ratio (Figure 2) of the accessions at midday can be attributed to higher midday PAR (Figure 8) experienced by the accessions. Since the accession exhibited full recovery of their darkadapted Fv/Fm ratio (Figure 3) by late afternoon, this decrease in the ratio can be associated with high carotenoid levels (Figure 5) which presumably dissipated excess excitation energy harmlessly as heat. The results are consistent with those of Jiang et al. [11] who observed that when plants are under high light Non-Photochemical Quenching (NPQ) protects the plants by dissipating excess light energy harmlessly as heat [20].
Previous studies have shown that plants have evolved several systems of reducing photodamage [6,10]. There is evidence in this study to suggest that protection of accessions to photodamage, was provided in part, by the accessions elevating their production of their antioxidant, viz., SOD and CAT (Figures 6 & 7) when they experience high photon fluxes (Figure 8). These results are consistent with those reported by Asada [13] and Jiang et al. [10] demonstrating that excess light can activate the production of Reactive Oxygen Species (ROS). Ghana and Tlokweng accessions exhibited higher levels of SOD and CAT than the Tsamaya and Tabala, which may indicate that they possess a good mechanism of scavenging ROS, thus protecting their photosynthetic machinery. Comparatively, the Ghana and Tlokweng accessions appeared to perform better than the Tsamaya and Tabala accessions as attested to by their efficient oxygen metabolizing enzymes (antioxidant) and higher carotenoids contents, when they experience higher PAR and high temperature [21].
It is therefore, concluded that the Tlokweng and Ghanaian accessions can be recommended for growing in Botswana, as a valuable feedstock and for the production of biodiesel, which is environmentally friendly.
References
- Divakara BN, Upadhyaya HD, Wani SP, Gowda C, Laxmipathi L (2010) Biology and genetic improvement of Jatropha curcas : a review. Appl Energy 87(3): 732-742.
- Openshaw KA (2000) A Review of Jatropha curcas: an oil plant of unfulfilled promise. Biomass Bioenergy 19(1): 1-15.
- Achten WMJ, Maes WH, Reubens B, Mathijs E, Singh VP, et al. (2010) Biomass production and allocation in Jatropha curcas seedlings under different levels of drought stress. Biomass Bioenergy 34(5): 667-676.
- Pandey VC, Singh K, Singh JS, Kumar A, Singh B, et al. (2012) Jatropha curcas: A potential biofuel plant for sustainable environmental development. Renewable and Sustainable Energy Reviews 16(5): 2870-2883.
- Gorji A (2015) A review on biodiesel production, key parameters in transesterification reaction, its effects on the environment and human health. Journal of Biodiversity and Environmental Sciences 7(3): 150-185.
- Juvany M, Muller M, Munné-Boch S (2013) Photo-oxidative stress in emerging and senescing leaves: a mirror image? Journal of Experimental Botany 64(11): 1093-1105.
- Massaoudou M, Abasse T, Rabiou H, Mahamane L (2020) Seasonal variation and modeling of leaf area growth in Jatropha curcas plants: Implication for understanding the species adaptation in the Sahel of Nigeria. African Journal of Plant Science 14(6): 205-212.
- Choinski JS, Johnson JM (1993) Changes in photosynthesis and water status of developing leaves of Brachystegis spiciformis Tree Physiology 13(1): 17–27.
- Greer DH, Halligan EA (2001) Photosynthetic and fluorescence light responses for kiwifruit (Actinidia deliciosa) leaves at different stages of development on vines grown at two different photon flux densities. Australian Journal of Plant Physiology 28(5): 373-382.
- Jiang CD, Li PM, Gao HY, Zou Q, Jiang GM, et al. (2005) Enhanced photoprotection at the early stages of leaf expansion in field-grown soybean plants. Plant Science 168(4): 911-919.
- Jiang CD, Gao HY, Zou Q, Jiang GM, Li LH (2006) Leaf orientation, photorespiration and xanthophyll cycle protect young soybean leaves against high irradiance in field. Environmental and Experimental Botany 55(1-2): 87-96.
- Niyogi KK, Björkman O, Grossman AR (1997) The roles of specific xanthophylls in Plant Biology 94(25): 14162-14167.
- Asada K (2006) Production and scavenging of reactive oxygen species in chloroplasts and their functions. Plant Physiology 141(2): 391-396.
- Tominaga J, Inafuku S, Coetzee T, Kawamitsu Y (2014) Diurnal regulation of photosynthesis in Jatropha curcas under drought during summer in a semi-arid region. Biomass and Bioenergy 67: 279-287.
- Maxwell K, Johnson GN (2000) Chlorophyll fluorescence - a practical guide. Journal of Experimental Botany 51(345): 659-668.
- Lichtenthaler K, Welburn AR (1983) Determination of total carotenoids and chlorophylls A and B of leaf extracts in different solvents. Biochemical Society Transactions 11(5): 591-592.
- Giannopolitis CN, Ries SK (1977) Superoxide Dismutases I. occurrence in higher plants. Plant Physiology 59(2): 309-314.
- Kar M, Mishra D (1976) Catalase, peroxidase and polyphenol oxidase activities during rice leaf senescence. Plant Physiology 57(2): 315-319.
- Chondrogiannis C, Kotsi K, Grammatikopoulos G, Petropoulou Y (2023) Seasonal differences in leaf photoprotective potential between adults and juveniles of two mediterranean perennials with distinct growth forms: A comparative field study. Plants 12(17): 3110.
- Demmig-Adams B, Adams WW III (1992) Photoprotection and other responses of plants to high light stress. Annual Review of Plant Biology and Plant Molecular Biology 43: 599-626.
- Zhang C, Bruins ME, Yang Z, Liu S, Rao P (2016) A new formula to calculate activity of superoxide dismutase in indirect assays. Analytical Biochemistry 503: 65-67.
© 2024 Hilary Shoniwa. This is an open access article distributed under the terms of the Creative Commons Attribution License , which permits unrestricted use, distribution, and build upon your work non-commercially.
 a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in
a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in 







.jpg)






























 Editorial Board Registrations
Editorial Board Registrations Submit your Article
Submit your Article Refer a Friend
Refer a Friend Advertise With Us
Advertise With Us
.jpg)






.jpg)














.bmp)
.jpg)
.png)
.jpg)










.jpg)






.png)

.png)



.png)






