- Submissions

Full Text
Modern Concepts & Developments in Agronomy
Phosphorus Nutrient in Organic Farming - A Review
Nguyen Hue*
Department of Tropical Plant and Soil Sciences, College of Tropical Agriculture and Human Resources, University of Hawaii, USA
*Corresponding author:Nguyen Hue, Department of Tropical Plant and Soil Sciences, College of Tropical Agriculture and Human Resources, University of Hawaii, USA
Submission: January 08, 2024;Published: January 24, 2024

ISSN 2637-7659Volume13 Issue 4
Abstract
Phosphorus (P) is essential to all living organisms and a major nutrient for successful crop production in organic farming. Organic production guidelines ban the use of highly soluble, manufactured P fertilizers, thus P sources for organic farming must come from P rock, green or animal manures, compost, and biofertilizers. In this article, we first briefly describe the P dynamics in soils, covering organic and inorganic P operational pools. Next, practical P sources for organic farming are discussed. These include phosphate rock, bone meal, animal and green manures, and compost. Bio-enhancers of soil P availability is covered next, including mycorrhizal fungi, P solubilizing microbes and biofertilizers with mixed microbial species. Detailed organic P molecules, such as phytate (monoesters phosphate) and nucleic acids (diester phosphates) and their hydrolyzing enzymes are presented. Finally, the role of organic P in crop growth is considered.
Keywords:Biofertilizers; Compost; Manures; Organic P; P Rock; Phosphatases; Phytate
Introduction
Organic farming follows a holistic approach to agriculture and avoids the use of synthetic fertilizers and pesticides [1]. More specifically, it relies on mined substances of low solubility (e.g., P rock, green sand), green and animal manures, compost, crop rotation, P or potassium (K) solubilizing microbes, and biofertilizers to maintain soil health and crop productivity [2]. With no chemical inputs, organic farming must be skillfully managed. Particularly, soil nutrient management poses a serious challenge to organic growing, especially if the cultivated soils are P deficient as is often the case of “old”, highly weathered soils in the Tropics [3,4].
Along with nitrogen (N) and K, P is an essential element and a major (needed in large amounts) nutrient to all crops [5]. The P requirement for optimal growth of most plants is in the range of 0.2-0.5% dry weight [6]. Biologically, P is a component of genetic molecules (DNA, RNA) as well as the molecule adenosine triphosphate (ATP), which transfers energy during photosynthesis and respiration [4,7,8]. ATP is an energy rich molecule, which can release approximately 30kJ mole-1 when transformed (phosphorylation) to ADP [4,6]. Thus, P is needed (and often found) in relatively large quantities in growing parts, seeds, and fruits; in fact, phytin is the principal storage form of P in seeds [9]. In P-deficient plants, reduction in leaf expansion and number of leaves is most noticeable, resulting in stunted growth [6,10]. The chlorophyll concentration tends to increase under P deficiency, and P-deficient leaves are dark green because leaf expansion is inhibited more strongly than chlorophyll formation [11]. In advanced stage of P deficiency, leaves turn purple [9,12].
Given the importance of P in plant nutrition and its low availability in highly weathered soils, the objective of this review was to better understand P transformation processes in soils so more efficient management practices can be realized, especially for organic farming.
The review utilized a thorough literature search with keywords such as sustainable agriculture, organic farming, crop nutrients, organic soil amendments, soil organic matter, soil phosphorus, organic P, biofertilizers, phosphohydrolases, P solubilizing microorganisms. Such comprehensive search was done for available electronic information resources in the Scopus, Web of Science and Science Direct databases. Most appropriate research and review studies were considered in this review.
Phosphorus Dynamics in Soils
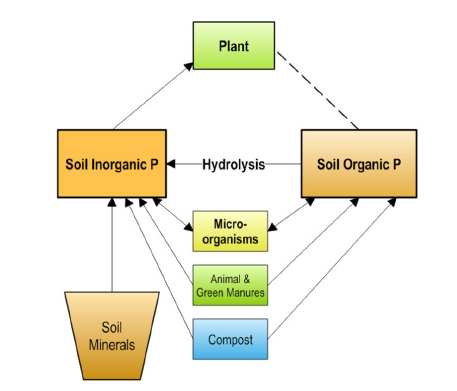
According to Sanchez [4], total P in soils has been used as a weathering index, meaning total P decreases with weathering stage. The author cited those representative soils from the US Midwest average about 3000 mg kg-1 total P in the topsoil (0-50cm depth), 500mg kg-1 in the more weathered soils of the Southeast, and about 200mg kg-1 in soils of the Tropics. Although total soil P only remotely reflects the actual P pools that affect crop growth, it illustrates a limited resource that we, as human beings, must face. Moreover, unlike N, P is not renewable since there is no atmospheric P reservoir. From the agricultural and environmental standpoint, total P in soil can be divided into five functional pools: (1) P in soil solution, (2) labile or weakly adsorbed P; (3) strongly adsorbed and/or precipitated P, including rock P, (4) organic P from manures, compost, bone meal, (5) microbial/biomass P (Figure 1). Pools 2 and 3 are inorganic solids; pools 4 and 5 are obviously organic.
Figure 1:Representation of the different P pools in soils. The different numbers refer to the processes affecting the P pools: (1) adsorption, (2) desorption, (3) dissolution, (4) precipitation, (5) assimilation, (6) mineralization, (7) decomposition, and (8) Phosphorus solubilization by microbes. Modified from Nesme et al. [16].
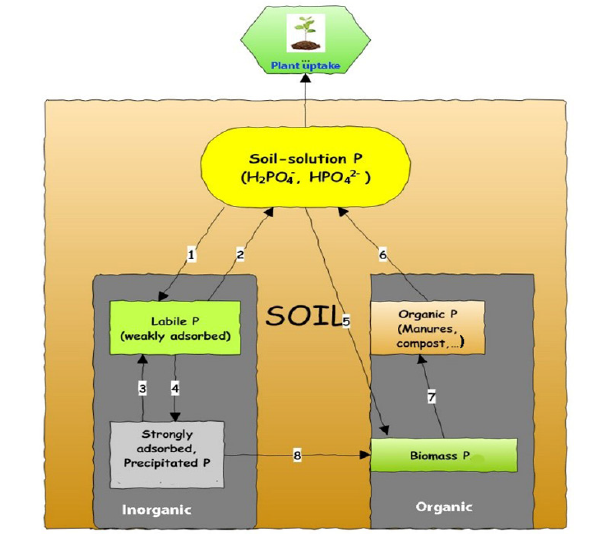
Soil-solution P pool is rather small. Its P concentration usually ranges from 0.1μM (or 0.003mg L-1) to 10μM (0.31mg L-1), with an average of 0.05mg L-1 [3,13,14]. Soil-solution P exists predominantly as H2PO4- anion at pH 3.0-7.0 and as HPO42- anion at pH > 7.2 [4,9,15]. Both anions move by the slow diffusion process and are readily taken up by plants. However, the dynamics of soil-solution P are fast and are interconnected with the other P pools [16].
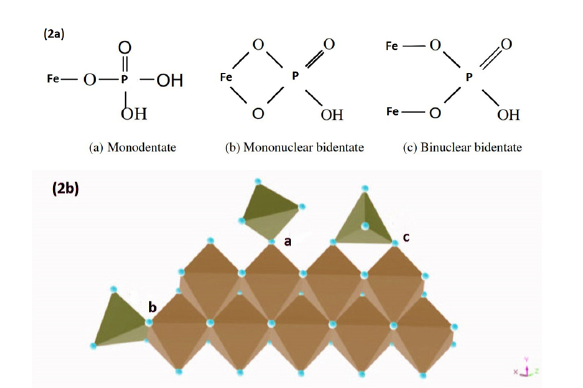
The weakly adsorbed or labile P pool and the strongly adsorbed or precipitated P pool are inorganic P attached to the soil solid phases, which may include (a) positively charged minerals (predominantly aluminum (Al) and iron (Fe) oxides, hydroxides), and some clay minerals, and (b) P-bearing minerals such as variscite [Al(OH)2H2PO4], strengite [Fe(OH)2H2PO4] in acidic environment, and hydroxyapatite [Ca5(OH)(PO4)3] in neutral or alkaline soils [4,16,17]. For example, Figure 2 shows the adsorption of H2PO4- ion on goethite (FeOOH) forming inner-sphere complexes. The formation of variscite mineral from H2PO4- ion and soil-solution Al3+ is shown below:
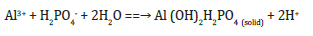
Figure 2:Adsorption of phosphate on soil Fe oxides 2a: 2D-sketches of possible complexes, 2b: 3D-sketches of phosphate adsorbed on goethite (FeOOH) mineral. Adapted from Celi et al. [18].
Although the inorganic pools are commonly dominant (>50%) in cultivated soils, in forest soils or under organic farming practices, organic P can be high, ranging from 30 to 80%, which includes P in crop residues, green and animal manures, and compost as well as P inside microbial tissues [18-20]. Most soil organic P compounds have P bonded to oxygen in ester bonds (C-O-P), unlike in soil organic N compounds where N is bonded directly to carbon (CN). Over 50% organic P is monoesters, mainly inositol phosphate (phytate); about 5% is phospholipids, and 2% is nucleic acids [21]. Phosphate ester bonds can be readily broken by phosphatases, which are extra-cellular enzymes and produced by certain bacteria, fungi, and plant roots [22].
Practical Phosphorus Sources for Organic Farming
Phosphate rocks
Since igneous and metamorphic P rocks are much less soluble than their sedimentary counterpart, only the latter is used as a P source in organic farming. Chemically, P rock is a combination of hydroxyapatite [Ca10(OH)2(PO4)6] and fluoroapatite [Ca10F2(PO4)6] with some minor substitution of OH- or F- by carbonate [4]. In the USA, Florida, North Carolina, Tennessee and Idaho are the states that have had P rock mining operations [23]. The solubility (and plant-availability) of rock P is quite low, so its use, especially in organic farming, requires some soil amending actions such as by keeping relatively low pH and/or low Ca2+ (Ca2+ can be lowered by chelation with organic-acid anions released from manures) as illustrated by the following chemical reactions.
Recently, Huang and Hue [3] reported that adequate levels of P nutrition for soybean (Glycine max L.) seedlings could be attained in an acidic Oxisol by adding finely ground Florida rock P at 75mg kg-1 (approximately 150kg ha-1 of rock P) and 1.0g kg-1 (or about 2 tons ha-1) of coral lime. The resulting pH was 5.2 and soil-solution P was 0.05mg L-1. The above reaction also provides the logic of using neutral ammonium citrate (or NAC at 2% concentration) to estimate the P availability of different P rocks. According to Sanchez [4], P rocks with greater than 5.9% P soluble in NAC can be applied directly to most annual and perennial crops. Those with 3.4-5.9% P soluble in NAC can be applied directly to low P-demanding crops like forage species, sugarcane (Saccharum officinarum) and tree crops. Those P rocks with less than 3.4% P soluble in NAC could be used on tea (Camellia chinensis L.) plants and perennial tree crops. A field study on two low-P, acid Ultisols in Indonedia [24] showed that a Moroccan P rock with high NAC extractable P (applied one time at approximately 130kg P ha-1) outperformed three other P rocks from Jordan, Senegal and Tunisia, and produced as much maize (Zea mays L.) yields as a simple superphosphate manufactured chemically during six growing seasons (3 years).
Fine sizes (< 100 mesh or 0.15mm) of P rock and its use in combination with other organic inputs such as manures or compost (which may release Ca chelating compounds) also help increase its P releasing capacity because of small sizes and thus large surface areas.
Bone meal (about 30% total P) and meat from cattle or fish (approximately 3 -5% P) can be used as a source of P in organic farming. These materials consist mainly of apatite-like calcium phosphate minerals, so they should react in a manner similar to rock P. As an example, Jeng et al. [25] reported that an application of 500 kg ha-1 of a meat-bone meal containing approximately 8% N, 5% P, and 10% K to a field experiment in Norway produced maximum yield of barley (Hordeum vulgare L.) based on the fact that a supply of extra mineral P gave no further yield increase.
Animal manures as Phosphorus sources in organic farming
Unlike P rocks, which may have 10-18% total P with low solubility and slow-release rate, P in manures is rather low (range: 1-5% P, average 2%) and the release rate is relatively fast [26]. Furthermore, manure P content varies significantly with animal species, feed, bedding, and manure storage practice (Table 1). For example, as calculated from Table 1, P content was 1.0% in beef manure and 4.4% in poultry layer manure. Manure P is quite water soluble; it is commonly accepted that about 70% of P in most animal manures are available to the crop during the year of application [26]. Beneficial effects of manure were documented in long-term (25 years) experiments in China showing that pig solid manure produced greater wheat (Triticum aestivum L.) and maize (Zea mays L.) yields than did synthetic fertilizers due mainly to improved soil fertility [27].
Table 1:Typical nutrient content from different animal manures [26].

In general, poultry manure has lower N/P ratios than cattle manure due to its higher P content. This may result in over P fertilization if amounts applied are to meet N requirements of crops [28].
Green manures: Manures from plants, even legumes, contain only 0.2-0.4% P [29], whereas their N can be as high as 6% [12,30]. Thus, green manures are more often used as an organic source for N than for P [31]. A quick estimation would show that at least 10 ton (dry weight basis) per hectare (ha) of a green manure having 0.2% P must be added to provide 20kg ha-1 of total P, which is a marginally sufficient quantity of required P for good plant growth, especially in P-poor soils such as Oxisols and Ultisols of the Tropics. Nevertheless, increases in P uptake as much as 2.8 times when a mixture of red clover (Trifolium pretense), hybrid lucern (Medicago media) and white meliot (Melilotus albus) was applied as green manure to a sandy loam soil of Estonia [32]. The P contribution from that mixed green manure was 17-24kg ha-1.
Compost: Since compost is often made from plant materials, either alone or in combination with animal manures, P in compost is also low, ranging between 0.1 and 2.3% [7]. Plant-based compost usually contains less nutrients (N, P, K) than animal manure-based compost [29]. In any case, compost has been used to replace chemical fertilizers in farming systems partially or completely [29]. Castan et al. [33] reported that applications of four types of compost at 40 tons ha-1 to a sandy soil of subtropical northeast Argentina resulted in increases in soil Olsen-P between 25% and 525% compared to a non-amended control. Eghball et al. [34] reported increases in soil Bray-P by 57% - 159% with 32 and 64kg ha-1 P applications, respectively, of a composted manure applied over a 4-year period.
Bio-Enhancers of Soil Phosphorus Availability
The available phosphorus in organically managed soils could be enhanced by manipulating plant root morphology, its rhizosphere composition and/or adding biofertilizers.
Given the low concentration of phosphate ions in the soil solution and their poor mobility, root architecture plays an important role in P acquisition [35]. It is no surprise that plants need to develop a large volume of the rhizosphere to access enough P, especially in low-P soils. Producing proteiod roots is a response to low P in some plant species such as macadamia (Macadamia integrifolia L.) [36]. Other plant species can exude P-mobilizing compounds such as protons, carboxylates, and phosphatases as triggered by P deficiency [13,37]. In addition, the rhizodeposition of carbon-rich compounds stimulates both naturally occurring and inoculated microorganisms in the rhizosphere, which can alter the availability of soil P [38,39].
Mycorrhizal fungi
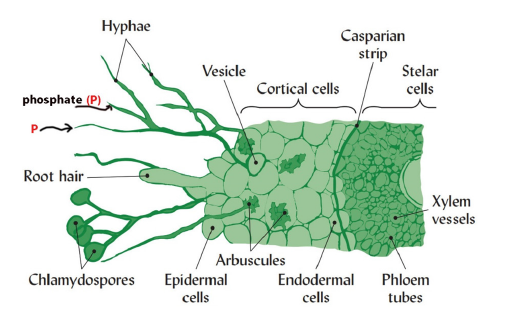
While root hairs can extend up to 1-2mm away from the root surface, mycorrhizal hyphae can extend beyond many cm [40], thus playing an exclusive role in obtaining remotely placed soil P. Mycorrhizae are, in fact, essential root extenders. They infect the roots of most vascular plants, effectively increasing the soil volume from which plants can take up nutrients and water. There are two main mycorrhizal types: ecto- and endo-mycorrhizae [4,41]. Ectomycorrhizae form a fungal mantle around the roots and are often associated with tree species [42]. Endomycorrhizae are associated more with crop plants. They are classified as arbuscular mycorrhizae, ericoid mycorrhizae, arbutoid mycorrhizae, monotropoid mycorrhizae, ectendo mycorrhizae, and orchid mycorrhizae [43], of which arbuscular one is most agronomically important. Their fungal hyphae penetrate the cortex of root cells, forming a structure shaped like a tree, called arbuscule. The hyphae grow and develop round fruiting spores outside the roots. These mycorrhizae markedly increase the uptake of immobile nutrients such as P, also Fe, copper and zinc (Figure 3). Through mycorrhizal hyphae, the inflow of phosphate ions can be many orders faster than their diffusion through soil [44]. As a result, mycorrhizal plants use P rock more efficiently than non-mycorrhizal plants. The association between mycorrhizae and plant can be obligate or facultative. In such association, the fungus is dependent on its host for photosynthates and energy, in return it supplies nutrients to its host. In organic farming, it has been recommended that seeds be inoculated with effective arbuscular mycorrhizae, to make more efficient use of soil P [45].
Figure 3:The association between arbuscular mycorrhizal fungi and plant roots. Adapted from Weil and Brady [51].
Phosphorus solubilizing microbes?
Although total P content may be large, most phosphates are in solid forms and unavailable for plant uptake. The P-solubilizing bacteria (PSB) such as Achromobacter, Micrococcus, Aerobacter, and Pseudomonas are common in nature. Their number, however, may vary with soil type and environmental conditions. As an example, Oliveira et al. [46] isolated, screened and evaluated 45 PSB isolates from maize rhizosphere soil of a P-deficient Oxisol of the Brazilian Cerrado Biome for use as potential microbial inoculants. The authors reported that two strains B17 and B5, identified as Bacillus sp. and Burkholderia sp., respectively, were the most effective, mobilizing 67% and 58.5% of the total P in Ca3(PO4)2 after 10 days of incubation. PSB can increase P availability to plants by secreting organic acids such as citric, malic, oxalic tartaric, gluconic, etc. [47]. The secreted acids can either directly replace solidly held phosphate anions by their conjugate anions (e.g., citrate, malate, etc.) or by dissolving P minerals via chelating Ca in P rock or Al/Fe in sesquioxide minerals (e.g., gibbsite, goethite) that can strongly adsorb phosphate [48]. Sundara et al. [49] reported that an application of P rock along with a PSB, Bacillus megaterium var. phosphaticum, to a sugarcane field increased sugar yield and juice quality by 12.6% and reduced P requirement by 25%. Zhang et al. [50] reported that two fungi species: Aspergillus neoniger and Talaromyces aurantiacus, isolated from the rhizosphere of moso bamboo (Phyllostachys edulis) grown in acid soils of China, can considerably solubilize recalcitrant P sources, namely Ca3(PO4)2, FePO4, and AlPO4, in this decreasing order [51].
To use phosphate from its organic forms, the P compounds must first be hydrolyzed by phosphatases (acid and alkaline enzymes), which are of plant or microbial origin [52]. Some fungi of the genera Aspergillus and Penicillium can efficiently cleave the C-O-P ester bond and release P for plant uptake (Table 2). Inositol penta- and hexa-phosphates, which are the major storage form of P in plant seeds, can be hydrolyzed by the enzyme phytase which is produced by fungi of genera Aspergillus, Emericella, Gliocladium, Penicillium, and Trichoderma [48,53].
Table 2:Release potential of P-mobilizing enzymes by some important fungi [48].

Biofertilizers
Biofertilizers consisting of bacteria and fungi that can solubilize solid P and/or hydrolyze organic P, have been formulated and commercialized [44,54]. They can have dry (solid) or liquid formulations to prolong microbial viability under variable conditions and to ensure their efficiency even under biotic and abiotic stresses; and their nutrient enhancing functions can goes beyond P, including K, silicon (Si), and even micronutrients. For P nutrient, biofertilizers often contain one or more of the following microbes: (1) phosphate solubilizers: Bacillus, Pseudomonas, Penicillium, Aspergillus, Trichoderma, Rhizobium, Burkholderia, Flabobacterium, and Pantoea [55]; and (2) phosphate mobilizers: Arbuscular mycorrhiza (including Glomas, Giga spora, Sclerocytis) and ectomycorrhizae such as Amanita, Boletus, Laccaria, and Pisolithus [54].
Potential Contribution of Organic Phosphorus to Soil Fertility and P Nutrition of Crops
Major forms of organic P in soils
The concentration of soil P that presents in organic forms can vary from a few mgkg-1 to over 500mg kg-1 soil, or approximately 20-80% of total P in soils [14,52,55,56]. Table 3 presents some specific values of various soil P forms. About half of the soil organic P is inositol phosphates, other organic P are phospholipids (0.5- 5%), and nucleic acids (0.2-2.5%) [19]. Major organic P groups are briefly described below.
Table 3:Forms of P in selected soils amended with organic wastes. Adapted from [28].

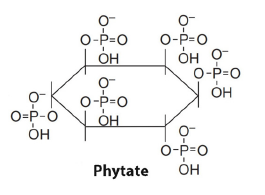
A. Inositol phosphates (monoester phosphates): The content of inositol phosphates is highly variable, but they are often the dominant component (up to 80%) of total organic P. Among the inositol hexaphosphates, there are several stereo isomeric forms, namely myo-, scyllo-, D-chiro-, and neo-inositol phosphates [52]. The myo-inositol hexakisphosphate (phytate, Figure 4) is the only compound found in soils, plants (mainly in seeds), animals, and microorganisms [52]. Inositol phosphates are more resistant to mineralization than the other fractions of soil organic P. This would explain the observation that they only account for 10-25% of fresh organic P inputs from microbial and plant sources, but commonly would accumulate into a major portion (over 50%) of organic P in soils. Furthermore, similar to inorganic phosphate ions, inositol phosphates can be strongly adsorbed by Al and Fe oxides in acid soils and can form precipitates with Ca in alkaline soils [18].
Figure 4:Chemical structure of myo-inositol hexakisphosphate (or phytate).
B. Phospholipids and nucleic acids (diester phosphates): Phospholipid concentrations vary from 0.2 to 14mg kg-1 soil, or about 0.5-5% with an average of 1% of soil organic P [52]. They may originate from microbes, plants or animals, but they decompose rather quickly in soil. Phosphoglycerides such as phosphatidylcholine and phosphatidylethanolamine can represent up to 40% of the phospholipids [57]. Less than 3% of soil organic P are nucleic acids and their derivatives [57]. Nucleic acids are rapidly mineralized, re-synthesized and combined with other soil constituents, or incorporated into microbial biomass [58]. Teichoic acids are bacterial polymers of glycerol phosphate linked to carbohydrates via phosphodiester bonds. They form the cell wall of Gram-positive bacteria.
C. Phophonates: Phosphonates have C-P bonds as opposed to C-O-P bonds in other organic P compounds found in soils. The strong C-P bonds make phosphonates resistant to chemical hydrolysis and thermal degradation. However, their concentrations in soils are very low, often non-consequential in terms of crop P nutrition. The most common and naturally occurring phosphonate is 2-aminoethylphosphonic acid, which can be produced by some organisms, including bacteria, fungi, and amoeba [59]. Some pesticides, such as glyphosate and phophinothricin, are synthetic phosphonates that may exist in agricultural soils.
Roles of enzymes and microorganisms in the mineralization of organic phosphorus
Organic P, be it derived from compost, animal manure or microbial biomass, must be converted (hydrolyzed) to inorganic P before it can be utilized by plants as illustrated in Figure 5.
Figure 5:Soil phosphorus dynamics: sources and transformations.
We will focus on the mineralization of Inositol phosphates because they are the main components of the phosphate monoesters (which represent 80% or more of the soil organic P). Phytate (myo-inositol hexakisphosphate) can be hydrolyzed by phosphatase enzymes [52]. Plant roots, fungi and bacteria can produce phosphatases in soils [47]. There are two types of phosphomonoesterase enzymes: the ‘acid’ phosphatases which show maximum activity in the acidic environment, and the ‘alkaline’ phosphatases which express maximum activity under alkaline conditions. Fungi Aspergillus terreus and A. niger and bacterium Bacillus subtilis can release phytases that are specific for phytate. The specificity of these enzymes, which belong to the histidine acid phosphatases, is mainly dependent on the amino acid residue 300 [60]. On the other hand, the alkaline phosphatase of the bacterium Lysobacter enzymogenes is nonspecific with the substrate used. It can hydrolyze glucose 6-phosphate and α- and β-glycerophosphates at rates similar to para-nitrophenylphosphate as well as phytate.
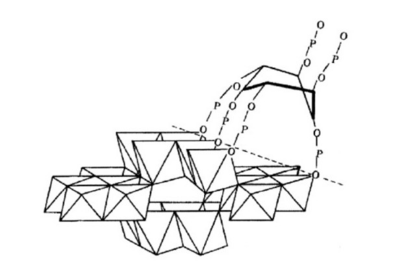
It should be noted that both enzymes and their substrates (organic P) are subject to adsorption and precipitation by the soil solid phases, particularly Fe and Al hydroxyl oxides and amorphous materials. According to Celi and Barberis [18], phytate can be adsorbed on goethite (FeOOH) via 4 of the 6 phosphate groups, forming Fe-O-P bonds (Figure 6), and via 2 phosphate groups on ferrihydrite. These adsorptive affinities are even stronger than those of these minerals with inorganic phosphate (H2PO4-).
Figure 6:Adsorption of phytate on the goethite (FeOOH) surface at pH 4.5. Adapted from Celi and Barberis [18].
In fact, the extent and rate of conversion of organic P into soluble or stable inorganic forms strongly depends on the nature of the original organic input, receiving soil (e.g., pH, clay type and content), as well as environmental factors, such as temperature and moisture. Generally, fresh plant residues, such as grass clipping or green manure, may quickly release P into the soil solution while more stable forms of organic inputs, such as aged animal manure or mature compost, act as slow-release P sources. The conversion rate from organic to inorganic P would be favorable and relatively fast if the ratio of its organic C to total P is < 200:1; if this C/P ratio is > 300:1, immobilization which is the incorporation of soluble P into microbial biomass may occur [4,28].
Organic P in soils, or P added to soils in manures or crop residues, represents an important source of P for plant growth [28]. Laboratory studies, using isotopic dilution techniques [61,62] reported that gross mineralization rates ranged from 0.2 to 4mg P kg-1day-1 (measured after 1-week equilibration), which are substantial in terms of crop P uptake. However, to be of direct and instant benefit to plants, mineralization of P must occur in close proximity to roots. This would allow plant roots to out compete microbial assimilation of P and overcome various edaphic chemical and physical reactions that lower soil-solution P concentration.
Furthermore, since extracellular phosphatase enzymes (produced by plant roots, soil microorganisms, and mycorrhizae) hydrolyze organic P to inorganic P, the production of these enzymes is positively correlated with the amount of available P in soils. As an example, Jiang et al. [63] reported that long-term (12 years) additions of organic fertilizers (oil cake, pig manure, or straw) elevated soil extracellular enzyme activities and increased yield and quality of tobacco (Nicotiana tabacum L.) grown on an Ultisol of Southeast China. The highest concentrations of total and available P were found in the pig manure treatment. Acid phosphatases were increased by all organic treatments. Organic P hydrolyzed by a commercial acid phosphomonoesterase also contributed significantly to the growth of slash pine (Pinus elliottii L.) seedlings grown on two forested Spodosols of Florida [64]. The authors further reported that NaHCO3- extractable P contained between 15 and 25% soluble organic P in the two A horizons, and over 75% organic P in the Bh horizons of the two soils. Yield and P uptake of agricultural crops, such as soybean and wheat, were significantly increased by applications (4, 8, and 16ton ha-1 year-1) of a cattle manure to an alkaline Vertisol in Bhopal, India [65]. During this 4-year field experiment, organic P (as sequentially extracted by NaHCO3 then NaOH) content was steadily built up from 78mg kg-1 in the no-manure control treatment to 103, 109, and 119mg kg-1 in the 4, 8, and 16ton ha-1 year-1 manure treatments, respectively [65].
Conclusion
Phosphorus is an essential nutrient to all crops. It must be present as inorganic P ions (H2PO4- and HPO42-) in soil solution before plants can take it up. Soil-solution P species are often low, usually below 0.10mgPL-1, and dynamically interact with both inorganic and organic P pools. The transformation between organic and inorganic P pools is governed by many complex factors, mainly by extracellular enzymes named phosphatases, which in turn are
References
- (2022) USDA National organic program. Standard manual. CCOF certification services LLC, Santa Cruz, California, USA.
- Soni R, Yadav SK (2019) Prospect of organic farming as financial sustainable strategy in modern agriculture. In: Panpatte DG, Jhala YK, (Eds.), Soil fertility management for sustainable development, Springer Nature Singapore Private Limited, Singapore, pp. 251-2665.
- Huang R, Hue N (2022) In transition towards organic farming: Effects of rock phosphate, coral lime, and green manure on soil fertility of an acid Oxisol and the growth of soybean (Glycine max L. Merr.) seedlings. Agriculture 12(12): 2045.
- Sanchez P (2019) Properties and management of soils in the Tropics. (2nd edn), Chapter 14, Phosphorus. Cambridge Univiversity Press, Cambridge, UK, pp. 370-414.
- Havlin J, Tisdale S, Nelson W, Beaton J (2017) Soil fertility and fertilizers. Chapter 5, Phosphorus. Pearson India Education Services, Ultar Pradesh, India, pp. 190-225.
- Hawkesford MJ, Cakmak I, Coskum D, De Kok LJ, Lambers H, et al. (2023) Functions of macronutrients. In: Rengel Z, Cakmak I, et al. (Eds.), Marschner’s mineral nutrition of plants, Academic Press, London, UK, pp. 201-281.
- Fageria NK (2009) The use of nutrients in crop plants. Chapter 3, Phosphorus. CRC Press, Boca Raton, Florida, USA, pp. 91-130.
- Barker A (2010) Science and technology of organic farming. Chapter 3: Requirements of plants for soil-derived nutrients. CRC Press, Boca Raton, Florida, USA, pp. 17-80.
- Sharanappa (2023) Soil fertility and nutrient management: Principles and practices. CRC Press, New India, Publishing Agency, New Delhi, India.
- Fageria NK, He ZL, Baligar VC (2017) Functions of phosphorus in crop plants. Phosphorus management in crop production. CRC Press, Boca Raton, Florida, USA, pp. 47-90.
- Chiera J, Thomas J, Rufty T (2002) Lead initiation and development in soybean under phosphorus stress. J Expt Bot 53(368): 473-481.
- Hue N, Silva J (2000) Organic soil amendments for sustainable agriculture. In: Silva JA, Uchida R (Eds.), Plant nutrient management in Hawaii’s soils. College of Tropical Agriculture & Human Resources, Univiversity of Hawaii at Manoa, Honolulu, USA.
- Hinsinger P (2001) Bioavailability of soil inorganic P in the rhizosphere as affected by root-induced chemical changes: A review. Plant & Soil 237: 173-195.
- Prasad R, Prasad S, Lal R (2017) Phosphorus in soil and plants in relation to human nutrition. In: Lal R, Stewart B (Eds.), Soil phosphorus. CRC Press, Boca Raton, Florida, USA, pp. 65-80.
- Strawn D, Bohn H, O’Connor G (2020) Soil Chemistry. (5th edn), John Wiley & Sons, Hoboken, New Jersey, USA, p.379.
- Nesme T, Colomb B, Hinsinger P, Watson CA (2014) In: Bellon S, Penvern (Eds.), Organic farming, prototype for sustainable agriculture. Springer Science, Dordrecht, The Netherlands, pp. 51-84.
- Lindsay L (1979) Chemical equilibria in soils. Chapter 12, Phosphates. John Wiley & Sons, New York, USA, pp. 162-209.
- Celi L, Barberis E (2005) Abiotic stabilization of organic phosphorus in the environment. In: Turner BL, Frossard E, et al. (Eds.), Organic phosphorus in the environment. CABI Publishing, Wallingford, UK, pp. 113-132.
- Tate KR (1984) The biological transformation of P in soil. Plant & Soil 76: 245-256.
- Shiau Y, Pai C, Tsai J, Liu W, Yam R, et al. (2019) Characterization of phosphorus in a toposequence of subtropical perhumid forest soils facing a subalpine lake. In: Qualls RG (Ed.), Carbon, nitrogen and phosphorus cycling in forest soils. Basel, Switzerland, pp. 170-183.
- Condron LM, Tiessen H (2005) Interactions of organic P in terrestrial ecosystems. In: Turner BL, Frossard E, et al. (Ed.), Organic phosphorus in the environment. CABI Publishing; Wallingford, UK, pp. 295-307.
- Asakawa S, Bunemann EK, Frossard E, Gregorich EG, Jansa J, et al. (2012) Phosphorus and sulfur in soil. In: Huang PM, Li C, et al. (Eds.), Handbook of soil sciences. (2nd edn), CRC Press, Boca Raton, Florida, USA, 1: 26.2-26.10.
- Mikkelsen R (2019) Sources of phosphorus for plants: past, present, and future. Better Crops 103(1): 17-21.
- Husnain, Rochayati S, Sutriadi T, Nassir A, Sarwani M (2014) Improvement of soil fertility and crop production through direct application of phosphate rock on maize in Indonesia. Procedia Engineering 83: 336-343.
- Jeng AS, Haraldsen TK, Gronlund A, Pedersen PA (2007) Meat and bone meal as nitrogen and phosphorus fertilizer to cereals and rye grass. In: Bationo A, Waswa B, et al. (Eds.), Advances in integrated soil fertility management in sub-Saharan Africa: Challenges and Opportunities, Springer, Dordrecht, The Netherlands, pp. 245-253.
- Shapiro CA, Johnson L, Schmidt AM, Koelsch R (2021) Determining crop available nutrients from manure. G1335. Soil management fertility, Nebraska Extension. University of Nebraska, Lincoln, Nebraska, USA.
- Cai A, Xu M, Wang B, Zhang W, Liang G, et al. (2019) Manure acts as a better fertilizer for increasing crop yields than synthetic fertilizer does by improving soil fertility. Soil and Tillage Research 189: 168-175.
- Pierzynski GM, Sims JT, Vance GF (2005) Soils and environmental quality. (3rd edn), CRC Press, Boca Raton, Florida, USA.
- Lazcano C, Decock C, Wong C, Garcia-Brucher K (2023) Assessing the effects of compost on soil health. In: Horwath W (Ed.), Improving soil health, Burleigh Dodds Science Publishing, Cambridge, UK, pp. 221-279.
- Uchida R (2000) Essential nutrients for plant growth: nutrient functions and deficiency symptoms. In: Silva JA, Uchida R, (Eds.), Plant nutrient management in Hawaii’s soils. College of Tropical Agriculture & Human Resources, University of Hawaii at Manoa, Honolulu, USA.
- Cherr CM, Scholberg JMS, McSorley R (2006) Green manure approaches to crop production: a synthesis. Agronomy J 98(2): 302-319.
- Talgre L, Lauringson E, Roostalu H, Astover A, Makke A (2012) Green manure as a nutrient source for succeeding crops. Plant Soil Environ 58(6): 275-281.
- Castan E, Satti P, Gonzalez-Polo M, Iglesias MC, Mazzarino MJ (2016) Managing the value of composts as organic amendments and fertilizers in sandy soils. Agriculture, ecosystems and environment 224: 29-38.
- Eghball B, Power JL (1999) Phosphorus- and nitrogen-based manure and compost applications: corn production and soil phosphorus. Soil Sci Soc Am J 63(4): 895-901.
- Lynch JP (2007) Roots of the second green revolution. Aust J Bot 55(5): 493-512.
- Hue N (2009) Iron and phosphorus fertilizations and the development of proteoid roots in macadamia. Plant & Soil 318: 93-100.
- Vance CP, Uhde-Stone C, Allan DL (2003) Phosphorus acquisition and use: critical adaptations by plants for securing a nonrenewable resource. New Phytol 157(3): 423-447.
- Guppy CN, McLaughlin MJ Z (2009) Options for increasing the biological cycling of phosphorus in low-input and organic agricultural systems. Crops Pasture Sci 60(2): 116-123.
- Richardson AE, Barea JM, McNeil AM, Prigent-Combaret C (2009) Acquisition of phosphorus and nitrogen in the rhizosphere and plant growth promotion by microorganisms. Plant & Soil 321: 305-339.
- Thonar C, Schnepf A, Frossard E, Roose T, Jansa J (2011) Traits related to differences in function among three arbuscular mycorrhizal fungi. Plant & Soil 339: 231-245.
- Smith SE, Read DJ (2008) Mycorrhiza symbiosis. (3rd edn), Academic Press, London, UK.
- Dominguez-Nunez JA, Berrocal-Lobo M, Albanesi AS (2019) Ectomycorrhizal fungi: role as biofertilizers in forestry. In: Giri B, Prasad R, et al. (Eds.), Biofertilizers for sustainable agriculture and environment, Springer Nature, Switzerland, pp. 67-82.
- Sharma R (2017) Ectomycorrhizal mushrooms: their diversity, ecology and practical applications. In: Varma A, Prasad R, et al. (Eds.), Mycorrhiza- function, diversity, state of the art, Springer, Berlin, Germany, pp. 99-131.
- Giri B, Prasad R, Q Wu, Varma A (2019) Biofertilizers for sustainable agriculture and environment, Springer Nature, Switzerland.
- Dai M, Schneider K, Cade-Menun B, Ferrera-Cerrato R, Hamel C. Arbuscular mycorrhiza and the phosphorus nutrition of organic crops. In: Martin R, MacRae R (Eds.), Managing energy, nutrients and pests in organic field crops, CRC Press, Boca Raton, Florida, USA, pp. 35-58.
- Oliveira CA, Alves VMC, Marriel IE, Gomes EA, Scotti MR, et al. (2009) Phosphate solubilizing microorgansisms isolated from rhizosphere of maize cultivated in an oxisol of the Brazillian Cerrado Biome. Soil Biol Biochem 41(9): 1782-1787.
- Richardson AE, George TS, Hens M, Simpson RJ (2005) Utilization of soil organic phosphorus by higher plants. In: Turner BL, Frossard E, et al. (Eds.), Organic phosphorus in the environment, CABI Publishing, Wallingford, UK, pp. 165-184.
- Tarafdar JC (2019) Fungal inoculants for native phosphorus mobilization. In: Giri B, Prasad R, et al. (Eds.), Biofertilizers for sustainable agriculture and environment, Springer Nature, Switzerland, pp. 21-40.
- Sundara B, Natarajan V, Hari K (2002) Influence of phosphorus solubilizing bacteria on the changes in soil available phosphorus and sugar cane and sugar yields. Field Crop Res 77(1): 43-49.
- Zhang Y, Chen F, Wu X, Luan F, Zhang L, et al. (2018) Isolation and characterization of two phosphate-solubilizing fungi from rhizosphere soil of moso bamboo and their functional capacities when exposed to different phosphorus sources and pH environments. Plos One 13(7): e0199625.
- Weil RR, Brady NC (2017) The nature and properties of soils. (15th global edn), Pearson Education, London, UK.
- Quiquampoix H, Mousain E (2005) Enzymatic hydrolysis of organic phosphorus. In: Turner BL, Frossard E, et al. (Eds.), Organic phosphorus in the environment, CABI Publishing, Wallingford, UK, pp. 89-112.
- Kaul S, Sharma S, Apra, Dhar MK (2019) Phosphate-solubilizing fungi. And their potential role in sustainable agriculture. In: Giri B, Prasad R, et al. (Eds.), Biofertilizers for sustainable agriculture and environment, Springer Nature, Switzerland, pp. 371-393.
- Wong CKF (2021) The CY. Impact of biofertilizers on horticultural crops. In: Inamuddin, Ahamed MI, et al. (Eds.), Biofertiliers: study and impact, Scrivener Publishing, Malaysia, pp. 39-104.
- Adetunji CO, Anani OA, Thangadurai D, Islam S (2023) Application of phosphate solubilizing microorganisms for effective production of next generation biofertilizer: A panacea for sustainable organic agriculture. In: Sangeetha J, Soytong K, et al. (Eds.), Organic farming for sustainable development, Apple Academic Press, Palm Bay, Florida, USA, pp. 77-103.
- Li T, Yao J, Zeng R, Chen Y, Hu L, et al. (2022) Characteristics of organic phosphorus pool in soil of typical agricultural systems in South China. Horticulturae 8(11): 1055.
- Dalal RC (1977) Soil organic phosphorus. In: Brady NC (Ed.), Advances in agronomy. Academic Press, New York, USA, 29: 83-117.
- Anderson G (1980) Assessing organic phosphorus in soils. In: Khasawneh FE, Sample KC, et al. (Eds.), The role of phosphorus in agriculture, Soil Sci Soc Am, Madison, Wisconsin, USA, pp. 411-431.
- Upadhayay VK, Sharma A (2022) Organic phosphorus forms in soil and their microbial mediated mineralization. Vigyan Varta 3(5): 9-12.
- Mullaney EJ, Daly CB, Kim T, Porres JM, Lei XG, et al. (2002) Site-directed mutagenesis of Aspergillus niger NRRL 3135 phytase at residue 300 to enhance catalysis at pH 4.0. Biological and biophysical Research Communications 297(4): 1016-1020.
- Oehl F, Oberson A, Sinaj S, Frossard E (2001) Organic phosphorus mineralization studies using isotopic dilution techniques. Soil Sci Soc Am J 65(3): 780-787.
- Lopez-Hernandez D, Brossard M, Frossard E (1998) P-isotopic exchange values in relation to Po mineralization in soils with very low P-sorbing capacities. Soil Biol Biochem 30(13): 1663-1670.
- Jiang Y, Zhang R, Zhang C, Su J, Cong W, et al. (2022) Long-term organic fertilizer additions elevate soil extracellular enzyme activities and tobacco quality in a tobacco-maize rotation. Frontiers in Plant Sci 13: 973639.
- Fox TR, Comerford NB (1992) Rhizosphere phosphatase activity and phosphatase hydrolysable organic phosphorus in two forested Spodosols. Soil Biol Biochem 24(6): 579-583.
- Reddy DD, Rao AS, Rupa TR (2000) Effects of continuous use of cattle manure and fertilizer phosphorus on crop yields and soil organic phosphorus in a Vertisol. Biores Techn 75(2): 113-118.
© 2024 Nguyen Hue. This is an open access article distributed under the terms of the Creative Commons Attribution License , which permits unrestricted use, distribution, and build upon your work non-commercially.
 a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in
a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in 







.jpg)






























 Editorial Board Registrations
Editorial Board Registrations Submit your Article
Submit your Article Refer a Friend
Refer a Friend Advertise With Us
Advertise With Us
.jpg)






.jpg)














.bmp)
.jpg)
.png)
.jpg)










.jpg)






.png)

.png)



.png)






