- Submissions

Full Text
Modern Concepts & Developments in Agronomy
Construction of VIGS Vector of Root-knot Resistant WRKY64 Gene in Rapeseed Addition Line EE
Ping Yu1, Lihua Zhao1, Chao Dong1, Xiaofang Zhang3, Hongmei Luo1, Holger Budahn2, and Shaosong Zhang1*
1Biotechnology and Genetic Germplasm Institute, Yunnan Academy of Agricultural Sciences, Kunming 650223, Yunnan, China
2Julius Kühn-Institut Institute for Breeding Research on Horticultural and Fruit Crops, Erwin-Baur-Straβe 27, 06484 Quedlinburg, Germany
3College of Life Science, Southwest Forestry University, Kunming 650223, Yunnan, China
*Corresponding author:Shaosong Zhang, Biotechnology and Genetic Germplasm Institute, Yunnan Academy of Agricultural Sciences, Kunming 650223, Yunnan, China
Submission: May 22, 2023;Published: June 26, 2023

ISSN 2637-7659Volume13 Issue 1
Abstract
The root knot nematode seriously harms the quality and yield of crops. The research of crop varieties resistant to root knot nematode has become one of the important ways to control this disease. Previous studies have found that WRKY64 gene in rapeseed addition line EE plays an important role in resisting Meloidogyne incognita. The construction of WRKY64 gene silencing vector provides material for further study on whether WRKY64 gene expression protein in rapeseed addition line EE plays an important role in resisting infection of M.incognita, and provides certain reference for breeding crop varieties resistant to root-knot nematode and green prevention and control of root-knot nematode. In this study, 156bp of WRKY64 gene was constructed into the pTRV-pTV00 gene silencing vector, and agrobacterium GV3101 was used to infiltrate the rapeseed addition line EE that had been inoculated with Meloidogyne incognita for 7 days. The recombinant vector pTRV-PTV00-WRKY64 of WRKY64 gene was successfully constructed, and the subsequent silencing efficiency test could be carried out. By RT-qPCR quantitative analysis, the silencing efficiency of WRKY64 gene expression was 80% after 9 days of inoculation with the silencing vector. WRKY64 gene silencing vector was successfully constructed and tested on the rapeseed addition line EE, providing material for the subsequent research on WRKY64 gene function and theoretical basis for the prevention and control of root-knot nematode disease.
Keywords:Rapeseed addition line; Root-knot nematodes; Virus-induced gene silencing; Real-time fluorescence quantitative PCR
Introduction
Root-knot nematode disease is becoming more and more serious in agricultural production, which occurs all over the world. Only in China, the annual crop reduction caused by root-knot nematode disease is 10%-30%, and more than 75% in serious cases, or even no crop. The pathogenic agent of this disease is root-knot nematode, [1]. Because of its wide host range, up to 3000 kinds of crops, it is considered to be the most invasive plant parasitic nematode, which has seriously damaged the agricultural production of rice, the main food crops in the world and the commercial crops, vegetables and fruits. Root-knot nematodes threatened the health of flowers, vegetables, fruits and coffee in Yunnan [2-4]. Therefore, root-knot nematode disease has become an important problem to be solved urgently. Recent studies have shown that Meloidogyne M.incognita and M.javanica are the most common species, and Meloidogyne M.incognita, M.javanica and M.arenaria are among the fastest spreading pests [5-6]. In this study, WRKY64 gene silencing vector was constructed to provide preliminary materials and theoretical basis for the subsequent functional study of this gene and green prevention and control of root-knot nematodes.
WRKY is a very important transcription factor in plants. It is easily stimulated by pathogens and disease-resistance hormones and can be induced to express WRKY. WRKY is widely involved in abiotic stresses such as salt tolerance, low temperature, drought and heavy metals, biological stresses such as verticillium wilt, bacterial wilt and black spot bacteria, physiological and biochemical activities such as growth and development and regulation of material metabolism. It plays an important regulatory role in plant resistance response and signal transduction pathway [7-8]. The involvement of WRKYs in regulating plant parasitic nematode infection has been studied in Arabidopsis, rice, tomato, pepper, and soybean. Ali et al. [9] showed that downregulation of WRKY gene in Arabidopsis Thaliana may be beneficial to nematode development by interfering with plant defense signals. After rice infection with M. graminicola, strong up-regulation of transcripts encoding OsWRKY62, OsWRKY59 and OsWRKY13 was detected [10]. Overexpression of three WRKY genes in soybean varieties susceptible to Heterodera glycines showed increased resistance to soybean-Heterodera glycines interactions [11]. SlWRKY45 plays an important role in signal transduction of plant root tissues that is conducive to the development of root-knot nematodes and can enhance sensitivity to nematodes [12]. SlWRKY3 acts as a positive regulator to induce anti-nematode invasion and infection response at the early stage of nematode infection [13]. Virus-Induced Gene Silencing (VIGS) technology has been used to study the functions of key genes under various stresses in many plants due to its advantages such as short experimental cycle, low cost, simple operation and no dependence on genetic transformation. After infecting plants with viruses carrying cDNA fragments of target genes, the degradation or methylation of endogenous target genes in plants is induced, and the target genes are silenced at the RNA level, thus causing changes in phenotype or physiological indicators, so as to study the function of target genes. This research has been applied to the study of functional genes related to resistance response, growth and development, and metabolic regulation of tobacco, tomato, wheat, rice and other plants [14-16]. Virus-induced silencing of SlWRKY72a and b genes in tomato resulted in significantly reduced Mi-1 mediated resistance to root-knot nematodes [17,18]. Atamian et al. [19] showed that after silencing SlWRKY70 gene in tomato, the disease resistance of Mi-1 gene against root node nematode was weakened, and Salicylic Acid (SA) up-regulated the transcription level of SlWRKY70, while methyl jasmonate inhibited the transcription level of SlWRKY70. These results indicated that SlWRKY70 could affect the function of Mi-1 gene against root-knot nematodes and play an important role in resistance to root-knot nematodes.
WRKY transcription factors play an important role in resistance to root-knot nematodes, but are mainly limited to a few model plants, such as Arabidopsis, tomato, and rice. The important role of WRKY transcription factor in the interaction between rapeseed and root-knot nematode is rarely reported. Previous proteomic based on label free showed that WRKY64 gene expression protein was specifically induced in rapeseed addition line EE, suggesting that WRKY64 gene might be involved in anti-root-knot nematode response of rapeseed addition line EE. VIGS is a quick and effective method to study plant gene function, but it has not been reported in rapeseed addition line EE. In this study, the silencing vector of WRKY64 gene in rapeseed addition line EE was constructed to study the anti M.incognita function of WRKY64 gene in rapeseed addition line EE, so as to prevent and control root-knot nematode disease with green. For decades, chemical pesticides have been successfully used to control root-knot nematode disease, but their use has been banned due to their harm to humans and ecology. Therefore, it is the most economical and effective measure to exploit natural resistance or resistance-related genes and cultivate resistant varieties to control root-knot nematode disease. In this study, Madora-EE and southern root-knot nematode were used as materials to construct WRKY64 gene of rapeseed addition line EE into TRV-pTV00 vector, and the silencing efficiency of this gene against M. incognita was analyzed absolutely by RT-qPCR. In order to further study the function of this gene, provide experimental materials for prevention and control of root-knot nematode disease.
Materials and Methods
Samples
In this study, Madora-EE was selected as plant material. The seeds were provided by Dr. Holger Budahn of the Julius Kühn- Institut, and sufficient plant materials had been obtained through the early planting work of this study. Meloidogyne incognita was provided by Professor Sun Ming’s research group at Huazhong Agricultural University.
Cultivation and inoculation of additional lines of rapeseed
Full seeds of the same size were selected, cleaned and soaked in ultra-pure water after surface disinfection with 0.1%NaClO, and placed overnight at room temperature. Seeds with basically the same growth were selected and planted in sand sterilized at high temperature and placed in a light incubator with a day/night temperature of 25/20 °C and light/darkness of 16/8h. Culture at 70% relative humidity until 3 to 4 leaves grow. J2 was inoculated in the rhizosphere of the plants with 1000 sticks per plant as the standard amount.
Total RNA extraction and WRKY gene sequence amplification of rapeseed addition lines EE
Total RNA was extracted from the leaves of rapeseed addition line EE according to the instructions of the polysaccharide polyphenol plant total RNA extraction kit of Tiangen Company. Using RNA as template, reverse transcription was performed using Tengen’s FastKing cDNA first strand synthesis kit (degenomics). Using the synthesized cDNA as template, according to the WRKY64 cDNA sequence, primer WRKY64-F:5’-CTAACACTCAGCCACACCGT-3’, WRKY64-R:5’-TCCTCCAAGGAAAACCGTCG-3’, was designed by NCBI. Extends some CDS fragments of WRKY64 gene. PCR reaction system: cDNA 2μL, 10×EasyTaq Buffer 2.5μL, dNTPs 2μL, forward primers and reverse primers 1μL each, Pfu Taq DNA enzyme 0.5μL, ddH2O supplement to total volume 25μL. PCR reaction conditions were 95 ℃ for 5min, 95 ℃ for 30 s, 67 ℃ for 30 s, 72 ℃ for 2min, 35 cycles. Extend for 10 min at 72 ℃. The amplified products were photographed by 1.0% agarose gel electrophoresis, 100V, 180mA, UVP gel imaging system.
Construction of VIGS carrier
The target fragment amplified by PCR was gelled and recovered using AXYGEN DNA gel recovery kit, then the pMD18-T vector was connected, and the connected plasmid pMD18-T-WRKY64 was transformed into the receptive cells of Escherichia coli DH5α according to the transformation instructions of DH5a. After screening by positive clones, the plasmid was extracted. Sent to biological company for sequencing verification.
The pMD18-T-WRKY64 plasmid and pTRV-PTV00 plasmid with correct sequences were double-digested with BamHI and HindIII restriction endonuclease, and the digested products were purified and recovered. The purified products were linked with T4 DNA ligase. Escherichia coli DH5α receptor cells were transformed, and the positive clones were screened and sent to Beijing Genomics Institute (BGI) for sequencing. The recombinant plasmid with correct sequence was the recombinant vector pTRV-PTV00- WRKY64 which was electrocuted into Agrobacterium.
Agrobacterium injection
The recombinant vector pTRV-PTV00-WRKY64 was transferred into Agrobacterium GV3101 by electroshock conversion. The transformed bacterial solution was screened on the resistant medium containing 50ug/mL kanamycin and 25ug/mL rifampicin, and PCR identified the positive clone bacterial solution as pTRVPTV00- WRKY64 Agrobacterium solution. Agrobacterium GV3101 strain pTRV-pBINTRA suspending bacteria liquid, which has been transferred into pBINTRA plasmid in equal volume, is fully mixed with pTRV-PTV00-WRKY64 suspending bacteria liquid to make injection infective solution for rapeseed addition line EE.
The treatment method in this study was to first inoculate rootknot nematode and then inject recombinant vector pTRV-PTV00- WRKY64. Healthy plants not inoculated with root-knot nematode were the blank control, and plants only inoculated with root-knot nematode were the positive control. After 10 days injection, the silencing efficiency was detected.
Detection of WRKY64 gene silencing efficiency
RT-qPCR was performed using Roche FastStart Universal SYBR Green Master kit. The reaction system was as follows: cDNA 0.4μL, forward primer and reverse primer 0.2μL, FastStart Universal SYBR Green Master 5μL, ddH2O 4.2μL. The reaction conditions: pre-denaturation at 95 ℃ for 3 min, denaturation at 95 ℃ for 10s, annealing at 55 ℃ for 20 s, extension at 72 ℃ for 30 s, 40 cycles. The recombinant plasmid pMD18-T-WRKY64 was diluted by 10 times continuous gradient, and the plasmid with 10 times difference concentration (10-1-10-6) was obtained. The standard curve of RTqPCR was established as the standard substance.
Silencing efficiency (%) = (C-t)/C×100 Where, C is the average copy number of WRKY64 gene in leaves inoculated with root-knot nematode. T is the average copy number of WRKY64 gene in leaves inoculated with silencing vector
Result
Cloning of WRKY64 gene fragment
When the additional line EE of rapeseed grew to 3-4 leaves, was inoculated with Meloidogyne incognita. A week after inoculation, the total RNA of leaves was extracted by kit, and the purity and concentration of RNA were detected by spectrophotometer. The A260/A280 readings of all samples were between 1.8-2.1, indicating that the RNA extracted was of high quality and met the requirements of subsequent experiments. cDNA synthesis by reverse transcription. WRKY64 gene fragments were amplified with specific priming WRKY64-F/WRKY64-R. A clear band with a size of about 200bp was obtained by 1.0% agar-gel electrophoresis, which was consistent with the expected size of WRKY64 gene. PCR amplification gel of WRKY64 gene (Figure 1). Purified and recovered target bands were connected to pMD18-T vector, and the recombinant plasmid was sent to BGI for sequencing. After DNAMAN sequence comparison, WRKY64 gene fragments were successfully cloned, which could be used to construct recombinant vectors.
Figure 1: PCR amplification gel of WRKY64 gene fragment and EE sample of healthy rape addition line. Marker: 2000bp, 1: WRKY64 gene.

Analysis of VIGS vector construction of WRKY64 gene
After double digestion, the recombinant plasmid pMD18- T-WRKY64 was connected to the pTRV-PTV00 vector to obtain the recombinant vector PTRV-PTV00-WRKY64. Agrobacterium tumefacie GV3101 receptor cells were transformed by electric shock method, and the 156bp target band was obtained by colony PCR amplification (Figure 2). Sequence comparison and analysis showed that it was completely consistent with the sequence of WRKY64 gene fragments, which proved that the recombinant vector pTRV-PTV00-WRKY64 gene was successfully constructed. Follow-up silencing efficiency tests can be conducted.
Figure 2: Digestion of WRKY64 gene fragments.
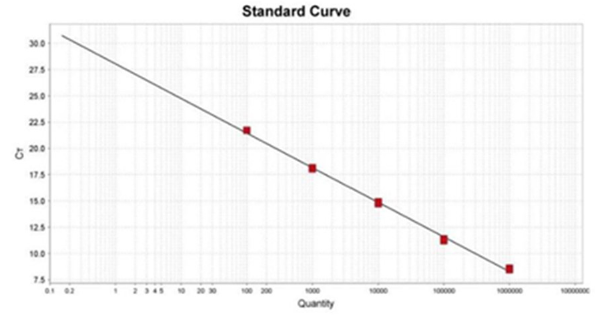
Establishment of RT-qPCR standard curve of WRKY64 gene
WRKY64 gene fragment was amplified by specific primers WRKY64-F/WRKY64-R. The fragment size was the same as expected, and the length was 156bp. The recombinant plasmid PMD18-TWRKY64 was obtained after the gene was purified and connected to pMD18-T vector. The plasmid concentration was 217.5mg·L-1 and the ratio of A260 to A280 was 2.09 by Nano Drop 2000. The purity met the requirements of RT-qPCR standard. Plasmid DNA with 5 standard gradients of 10-2-10-6 was used as template for amplification. After the reaction, the RT-qPCR standard curve and amplification curve of the samples were automatically obtained. The equation of the standard curve was Y = -3.366X + 34.58, the amplification efficiency was 99.321%, and the correlation coefficient was 0.9914. The Ct value in the standard curve showed a good linear relationship with the standard plasmid concentration, which could be used for quantitative analysis (Figure 3).
Figure 3: RT - qPCR labeling curve of WRKY64 gene.
Detection of WRKY64 gene silencing effect
The expression level of WRKY64 gene was detected by RT-qPCR to determine the silencing efficiency of WRKY64 gene. The results showed that the expression level of WRKY64 gene in the addition line EE was significantly decreased compared with the positive control inoculated with only Meloidogyne incognita without any vector injection. The expression level of WRKY64 gene in the addition line EE did not change compared with the blank control that was not inoculated with Meloidogyne incognita nor injected with any vector. The silencing efficiency of WRKY64 gene was 80%, indicating that the constructed pTRV-PTV00-WRKY64 vector could be used for the subsequent functional study of this gene.
Discussion
Rapeseed addition line EE is from oil radish E chromosome is attached to the rapeseed variety Madora by distant hybridization, and it exists stably as diploid in rapeseed [20-21]. Early resistance test results showed that Madora was susceptible to south root-knot nematode, and rapeseed addition line EE was highly resistant to South root-knot nematode [22]. Proteomic Lable-free techniques were used to analyze the differentially expressed proteins of Madora and EE in rapeseed during their interaction with affinity and non-affinity nematodes. The results showed that WRKY DNA-binding protein 64 (encoded by WRKY64 gene) was only specifically expressed in rapeseed addition line EE after infection by M.incognita.
WRKY transcription factor plays an important role in regulating plant growth and development and responding to various environmental stresses [23]. The functions of WRKY transcription factors in rapeseed were mainly studied by constructing overexpression vectors and CRISPR/Cas9 gene editing vectors. Chen et al. [24] overexpressed BnWRKY149 and BnWRKY217, which proved that BnWRKY149 and BnWRKY217 played an important role in the resistance to abiotic stress ABA [23]. Cui et al. [25] found that the overexpression of phosphate BnaWSR1 (BnaWSR1ca) in rapeseed protoplasts triggered the production of Reactive Oxygen Species (ROS) and cell death, while its ectopic expression in Arabidopsis increased the levels of salicylic acid (SA) and ROS, thus accelerating leaf senescence. Bioinformatics analysis showed that WRKY DNA-binding protein 64 belongs to the third class of WRKY transcription factors, which may be involved in the response to herbivores, pathogens, and nematodes.
Virus-Induced Gene Silencing (VIGS) is a genetic technique that inhibits endogenous gene expression in plants by inserting a recombinant virus with a target gene fragment. It is regarded as a powerful tool to study plant gene function. In the era of functional genome research, it is urgent to establish a technology platform for rapid identification of target sequence or gene function. Therefore, based on the protein level study, this study constructed a VIGS vector of WRKY64 gene and detected WRKY64 gene expression by RT-qPCR to determine the silencing efficiency of WRKY64 gene, which further indicated that WRKY64 gene in rapeseed additional line EE had function against Meloidogyne incognita. It will lay a theoretical foundation for disease resistance breeding in subsequent agricultural production and provide a theoretical basis for field green prevention and control of root-knot nematodes [25].
Acknowledgement
The authors acknowledge financial support from Yunnan Fundamental Research Projects (202101AT070268).
References
- Abad P, Gouzy J, Aury JM, Sereno PC, Danchin EGJ, et al. (2008) Genome sequence of the metazoan plant parasitic nematode Meloidogyne incognita. Nat Biotechnol 26(8): 909-915.
- Han BJ, ZhangLJ, Zhang J (2021) Research progress on control of root-knot nematode disease in crops. Yangtze River vegetables 22: 44-48.
- Hu XQ, Lu JF, Lin LF (2003) Discovery of shortscape fleabance root-knot disease in Yunnan. Journal of Yunnan Agricultural University 3: 317-319.
- Zhou YL, Yang W, Yu GH, et al. (2005) Species of megranate root-knot nematode from Yunnan Province in China. Journal of Huazhong Agricultural University 24(4): 351-354.
- Wang YF, Chen J, Zhang CX (2018) Investigation of occurrence and damages of major banana diseases and pests in dry-hot valley area of Lujiangba. Tropical Agricultural Sciences 38: 87-92.
- Janssen T, Karssen G, Verhaeven M, Coyne D, Bert W (2016) Mitochondrial coding genome analysis of tropical root-knot nematodes (Meloidogyne) supports haplotype based diagnostics and reveals evidence of recent reticulate evolution. Sci Rep 6: 22591.
- Karuri HW, Olago D, Neilson R, Mararo E, Villinger J (2017) A survey of root-knotnematodes and resistance to Meloidogyne incognita in sweet potato varieties from Kenyan fields. Crop Prot 92: 114-121.
- Li W, Li DH, Li HY, Wang MC, Wang Z, et al. (2023) The tomato WRKY transcription factor SlWRKY17 positively regulates drought stress tolerance in transgenic tobacco plants 69: 154.
- Goyal P, Devi R, Verma B, Hussain S, Arora P, et al. (2023) WRKY transcription factors: evolution, regulation, and functional diversity in plants. Protoplasma 260(2): 331-348.
- Ali MA, Wieczorek K, Kreil DP, Bohlmann H (2014) The beet cyst nematode Heterodera schachtii modulates the expression of WRKY transcription factors in syncytia to favour its development in Arabidopsis roots. PLoS One 9(7): e102360.
- Nguyễn PV, Bellafiore S, Petitot AS, Haidar R, Bak A, et al. (2014) Meloidogyne incognita-rice (Oryza sativa) interaction: a new model system to study plant-root-knot nematode interactions in monocotyledons. Rice 7(1): 23.
- Yang Y, Zhou Y, Chi Y, Fan B, Chen Z (2017) Characterization of soybean WRKY gene family and identification of soybean WRKY genes that promote resistance to soybean cyst nematode. Sci Rep-Uk 7: 17804.
- Chinnapandi B, Bucki P, Braun Miyara S (2017) SlWRKY45, nematode-responsive tomato WRKY gene, enhances susceptibility to the root-knotnematode; javanica infection. Plant Signal Behav 12(12): e1356530.
- Chinnapandi B, Bucki P, Fitoussi N, Kolomiets M, Borrego E, et al. (2019) Tomato SlWRKY3 acts as a positive regulator for resistance against the root-knot nematode Meloidogyne javanica by activating lipids and hormone-mediated defense-signaling pathways. Plant Signal Behav 14(6): 1601951.
- Hao MY, Hang Q, Shi GY (2022) Application and prospect of virus-induced gene silencing in crop gene function research. China Agricultural Science and Technology Review 24(1): 1-13.
- Li J, Luo JH, Yang P (2021) Research advances of applying virus-induced gene silencing in vegetables. Chinese Agricultural Sciences 54(10): 2154-2166.
- Guo Y, Liu ZD, Kang LR (2023) Optimization of efficient silencing system of tomato VIGS based on PDS gene. Crop Magazine 2: 46-50.
- Bhattarai KK, Atamian HS, Kaloshian I, Eulgem T (2010) WRKY72-type transcription factors contribute to basal immunity in tomato and Arabidopsis as well as gene-for-gene resistance mediated by the tomato R gene Mi-1. Plant J 63(2): 229-240.
- Atamian HS, Eulgem T, Kaloshian I (2012) SlWRKY70 is required for Mi-1-mediated resistance to aphids and nematodes in tomato. Planta 235(2): 299-309.
- Bakshi M, Oelmuller R (2014) WRKY transcription factors: jack of many trades in plants. Plant Signal Behav 9(2): e27700.
- Peterka H, Budahn H, Schrader O, Ahne R, Schutze W (2004) Transfer of resistance against beet cyst nematode from radish (Raphanus sativus) to rapeseed (Brassica napus) by monosomic chromosome addition. Theor Appl Genet 109(1): 30-41.
- Budahn H, Schrader OS, Peterka H (2008) Development of a complete set of disomic rapeseed-radish chromosome-addition lines. Euphytica 162: 117-128.
- Zhang SS, Schiiephake E, Budahn H (2017) Chromosomal assignment of oil radish resistance to Meloidogyne incognita and javanica using a set of disomic rapeseed-radish chromosome addition lines. Nematology 16: 1119-1127.
- Chen H, Wang YF, Liu J, Zhao T, Yang C, et al. (2021) Identification of WRKY transcription factors responding to abiotic stresses in Brassica napus Planta 255(1): 3-3.
- Cui X, Zhao PY, Liang WW, Cheng Q, Mu B, et al. (2020) A rapeseed WRKY transcription factor phosphorylated by CPK modulates cell death and leaf senescence by regulating the expression of ROS and SA-synthesis-related genes. Journal of Agricultural and Food Chemistry 68(28): 7348-7359.
© 2023 Shaosong Zhang. This is an open access article distributed under the terms of the Creative Commons Attribution License , which permits unrestricted use, distribution, and build upon your work non-commercially.
 a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in
a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in 







.jpg)






























 Editorial Board Registrations
Editorial Board Registrations Submit your Article
Submit your Article Refer a Friend
Refer a Friend Advertise With Us
Advertise With Us
.jpg)






.jpg)














.bmp)
.jpg)
.png)
.jpg)










.jpg)






.png)

.png)



.png)






