- Submissions

Full Text
Modern Concepts & Developments in Agronomy
Food Safety Issues in Semi-moist/Intermediate Moisture Foods and their Mitigation Using Clean Label Antimicrobials- A Review
Aiswariya Deliephan, Bhadriraju Subramanyam* and Charles G Aldrich
Department of Grain Science and Industry, USA
*Corresponding author: Bhadriraju Subramanyam, Department of Grain Science and Industry, Kansas State University, Manhattan, Kansas 66506, USA
Submission: February 21, 2023;Published: March 10, 2023

ISSN 2637-7659Volume12 Issue 4
Abstract
Semi-moist or intermediate moisturized foods with water activity in the range of 0.60-0.85 are primarily susceptible to mold contamination and mite infestation. Additionally, cross contamination of pathogenic bacteria from food contact surfaces can occur in processing facilities. Synthetic preservatives, fumigants and chemical sanitizers had been traditionally used to mitigate these contaminants; however, they could be potentially unsafe for health and environment in the long term. There is an increasing demand from consumers for natural and clean label antimicrobials to replace these synthetic components. This paper presents a review of food safety issues in semi-moist foods and some of the natural and clean label mitigation strategies to enhance food safety and shelf-life.
Keywords: Intermediate moisture food; Semi-moist food; Antimicrobial; Clean label
Introduction
Semi-moist or intermediate moisture foods have gained more attention worldwide by having features very similar to fresh food products, but with a longer shelf life [1]. By definition, semi-moist foods are shelf-stable food products that have water activities (aw) of 0.60-0.84, with a moisture content ranging from 15-40% and are edible without rehydration [2]. Production of semi-moist foods with properties close to fresh foods yet having extended shelf life to satisfy the demand of the consumer is vitally important for the food industry.
Semi-Moist Foods
Historically, ancient civilizations produced semi-moist/intermediate moisture foods using methods such as sun drying, smoking or roasting over fire, and adding salt to preserve food for winter months [3]. In the current times, foods are processed to semi-moist levels mostly by partial drying (e.g., dried fruit), osmotic drying using humectants (e.g., pickled vegetables), and food formulations using humectants and additives to keep them shelf stable (e.g., cakes, chewy dog treats). Many types of foods that are commercially available, like confectionery and pet foods, are specially formulated to achieve water activity in the semi-moist or semi-moist range (0.60-0.84 aw). Food ingredients are mixed with salt and/or sugar, and additives such as polyols (e.g., propylene glycol) and mold inhibitors (e.g., potassium sorbate) and then, subjected to processing methods such as cooking, extrusion, baking or dehydration to result in a semi-moist final product [4]. This method of formulating foods is fast and energy efficient and offers great flexibility in formulation.
The unique features that make semi-moist foods appeal to consumers include microbial safety, desirable odors, high nutritional values, and Ready to Eat (RTE) characteristics [5]. Some of the popular examples of semi-moist foods consumed in our day-to-day lives are fruit cake, country-style ham, fondants, high sugar cakes, sweetened condensed milk, salted fish, molasses, jams, pet food, dried fruits and nuts, parmesan cheese, chocolate bars, marshmallows, and biscuits. As these semi-moist food products encompass a wide category of foods ranging from cereal-based to meat-based to dairy products to pet foods, research in this area of foods contributes tremendously to the well-being of humans and pets all over the world.
Semi-moist pet foods (e.g., chewy dog treats) are a smaller but significant portion of the manufactured pet food market, and they require the use of humectants and acidification to control water activity and mold growth. Semi-moist pet foods also have a low fiber content and relatively high sugar content, which make them highly palatable for pets and increasingly purchased by pet owners [6]. Semi-moist pet foods are manufactured in a way similar to extruded food, but the water content is maintained at a higher level because of the added humectants. The final moisture content of 25 to 35% is more prone to mold growth and spoilage, which is mitigated by mold or bacterial inhibitors as well as managing the ‘active water’ component of the food [6]. The amount of water activity is a measure of the amount of water available for bacterial growth and the addition of humectants helps to keep this at a low level, which effectively inhibits their growth despite a higher total water content [6]. It is apparent that much effort is put toward producing products that not only meet nutrient targets but that are also safe for their intended purposes. In addition to the care paid to details during the formulation and manufacturing process, companies maintain post manufacturing quality controls that further ensure safety and nutritional adequacy for the products. Post manufacturing quality control consists of nutrient testing post processing to determine nutrient losses, long- and short-term storage of foods to assess microbial stability and shelf-life losses of nutrients, as well as ongoing tracking of product stability in multiple environmental conditions [6].
Food Safety Issues in Semi-Moist Foods
Semi-moist food products are below the minimum water activity for most bacteria, which require 0.90aw, but are susceptible to yeast and mold growth which leads to spoilage. Lowering the water activity is an essential processing step and the main microbial hurdle in semi-moist food products. However, setting the aw at a certain level cannot by itself ensure shelf stability. Other factors and properties of food systems should be considered, and often additional measures must be taken into account to achieve the desired stability. Table 1 shows some of the common microorganisms growing in the aw range of semi-moist foods [7].
Microbial stability is also the primary criterion for the viability of a semi-moist food product under development. Inhibiting microbial growth on a given substrate is not achieved exclusively by lowering the aw, but rather, it is a function of all contributing hurdles i.e., aw, pH, temperature, oxidation reduction potential, preservatives, and existing microflora [8]. Numerous microorganisms of significance that cause spoilage have been shown to be able to grow at aw in the range of 0.60 to 0.84 when other conditions are favorable (Table 1). Thus, additional precautions, besides the adjustment of aw, must be sought to inhibit or limit the proliferation of these microorganisms in semi-moist food products.
Table 1: Microorganisms growing in the aw range of semi-moist foods.
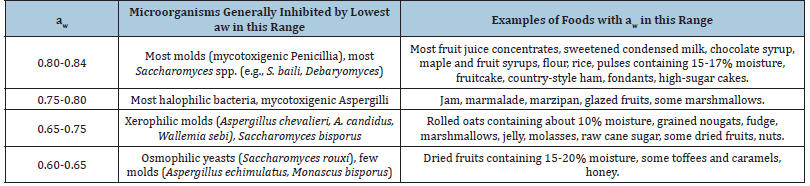
The pathogenic microorganisms of major concern in foods are effectively inhibited by the reduction of aw to the intermediate moisture zone (0.60-0.84aw). For example, the growth of Clostridium spp. were prevented by reduced aw regardless of storage temperature and pH [9]. However, because growth could potentially occur during formulation and storage prior to the reduction of aw, good hygienic and manufacturing practices are essential. Bacillus spp. require a minimum aw of 0.89 to 0.90 for growth [10]. At water activities found in semi-moist foods, Salmonella spp. cannot multiply due to a limit for growth of 0.95aw, but their resistance to heat is greatly increased, and they may persist in semi-moist foods for long periods [11]. Listeria monocytogenes Pirie (Listeriaceae: Bacillales), can grow at considerably lower aw with the reported limit of growth at 0.92aw [12,13]. The food pathogen able to grow at even lower aw is Staphylococcus aureus Rosenbach (Staphylococcaceae: Bacillales), which has also been shown to grow at aw above 0.84 to 0.85 if the pH is favorable. Formulation of semi-moist food products at the highest possible moisture content, for improved texture and palatability, requires additional measures for the inhibition of S. aureus. The same is true for molds. The most often encountered molds are Aspergillus and Penicillium spp., which can grow at aw above 0.77 to 0.85. The minimum aw for mycotoxin production by these molds is usually higher. Several xerophilic and xero-tolerant molds can grow at aw down to 0.62 to 0.64. Yeast growth is another potential problem with semi-moist foods. Compared with the most tolerant halophilic bacteria that can grow down to the aw levels of saturated sodium chloride (i.e., aw 0.75), osmophilic yeasts can grow at water activities down to 0.62aw (e.g., Zygosaccharomyces rouxii (Boutroux) Yarrow (Saccharomycetaceae: Saccharomycetales). Good manufacturing practices, pasteurization of mixtures, and use of chemical preservatives, such as sulfites, benzoates, parahydroxybenzoates, sorbates, and diethyl pyrocarbonate, are the usual control measures in semi-moist foods [3].
Apart from the above-mentioned food safety and spoilage issues of bacteria, molds and yeasts, another important issue for semi-moist foods during storage is infestation by mites. Mites are small arachnids (arthropods) that feed on stored food products causing economic losses and may also cause allergies to humans and pets [14]. They also act as vectors of other pathogens [15]. Storage mites (e.g., Acarus siro Linnaeus (Acaridae: Sarcoptiformes) and Tyrophagus putrescentiae Schrank (Acaridae: Sarcoptiformes) feed on mold that grows on food [16]. Storage mites thrive in environments where there is moisture or increased humidity but are most frequently found in dry semi-moist food items such as flour, grains, dried fruits, cereal, and semi-moist dog and cat foods.
Molds and mycotoxins affecting semi-moist foods
Fungi are widely distributed in the environment and are frequent contaminants of food and animal feed. They appear as spoilage-causing organisms under reduced values of water activity in intermediate (0.75-0.90 aw) and low moisture (<0.75aw) foods, and foods with low pH values <4.0 (acidic foods). The minimum water activity limits for the growth of some of the common mold species found in semi-moist foods are summarized in Table 2 [17].
Table 2:Minimum water activity limits for the growth of common mold species found in semi-moist foods.
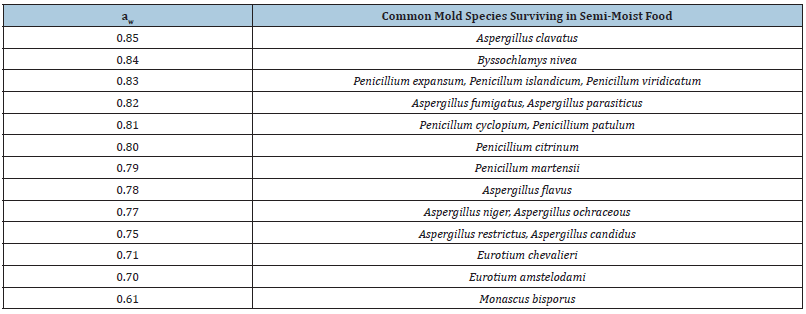
The most common species of fungi isolated from human foods belong to the genera Aspergillus, Penicillium, Fusarium, Alternaria, Cladosporium, Mucor, Rhizopus, Eurotium and Emericella [18,19]. Species of genera Aspergillus, Penicillium and Eurotium are storage fungi that can develop at ≤0.85 aw, so they can be isolated from spices, dried fruits and vegetables, pumpkin seeds, sunflower seeds, stored cereals, and similar products that are under the categories of intermediate or low moisture foods [20]. Species of the genera Fusarium and Alternaria are ‘field’ fungi and their development requires higher moisture content in the substrate and lower temperatures [20]. These species are usually found in cereal grains and cereal products. Also, they are common causes of illness in fruits and vegetables in the field, in addition to species of the genera Sclerotina, Bortrytis, Monilia, Rhizopus, Mucor and Penicillium. Fungi are common contaminants of meat and milk products during storage, with species of genera Penicillium, Aspergillus, Cladosporium, Geotrichum, Mucor, Sporotrichum, Trichoderma commonly isolated from these food groups [20]
Growth of fungi in food leads to food spoilage, causing economic losses due to food waste. On the other hand, toxin-producing species of genera Aspergillus (A. carbonarius, A. flavus, A. ochraceus, A. oryzae, A. parasiticus, A. versicolor), Penicillium (P. nordicum, P. expansum, P. viridicatum, P. verrucosum), Fusarium ( F. culmorum, F. graminearum, F. oxysporum, F. verticillioides, F. proliferatum), Alternaria ( A. alternata, A. solani, A. brassicae, A. tenuissima, A. tomato) as well as teleomorphs of the class Ascomycetes (Petromyces alliaceus, Emericella nidulans, etc.) can biosynthesize toxic secondary metabolites namely mycotoxins. Examples of mycotoxins are aflatoxins, ochratoxin A, sterigmatocystin, patulin, fumonisins, zearalenone, deoxynivalenol, alternariol, alternariol monomethyl ether, and tenuazonic acid [18,19]. Intake of mycotoxins by animals and humans causes intoxication called mycotoxicosis. Mycotoxicosis leads to acute and chronic toxicity (cytotoxicity, hepatotoxicity, neurotoxicity, teratogenicity, mutagenicity, and carcinogenicity) [21]. At a cellular level, mycotoxins react with nucleic acids and inhibit the biosynthesis of macromolecules DNA and RNA, or act on structures and functions of biological membranes or impair the energy metabolism [22,23].
Molds and mycotoxins also affect pet food. A study by Beuno et al. [24] identified commonly occurring molds in 21 pet foods. These included dry dog kibbles across 8 commercial brands produced in Argentina. Molds were comprised mainly of Aspergillus, followed by Rhyzopus and Mucor spp., and A. flavus was found in 14 of 60 pet food samples purchased in Portugal [25]. In another study Scudamore et al. [26] identified significant growth of Aspergillus spp. in commercial pet food with 20-25% moisture content after 4 weeks of incubation. Although the presence of toxigenic fungi does not necessarily result in mycotoxin production, preventing fungal growth in pet food can certainly minimize the risk of mycotoxicosis. Fungal contamination can lead to economic losses associated with nutrient and palatability reduction, and the presence of mycotoxins affects both animal and human health [27]. Aspergillus flavus is the most reported in pet food and responsible for the production of aflatoxins.
Aflatoxins are a group of mycotoxins produced by Aspergillus spp., mainly A. parasiticus and A. flavus [28]. They are common fungal contaminants of corn, peanuts, cottonseed, tree nuts, wheat, and rice. Aflatoxins B1, B2, G1, and G2 are the four naturally occurring forms of aflatoxins, with aflatoxin B1 the most potent, prevalent, and carcinogenic [29]. Aflatoxins are hepatotoxic and carcinogenic. Dogs are extremely sensitive to this group of toxins, with the liver being their main target [30]. Dogs exposed to 0.5-mg of aflatoxin/kg of body weight typically die within days, exhibiting vomiting, depression, polydipsia, polyuria, and hepatitis [31].
In the US aflatoxicosis related illnesses in dogs and Aspergillus flavus contaminated dog food recalls were reported in 2005. More recently, in 2020, 28 deaths and 8 illnesses were reported in dogs that consumed the recalled Sportmix™ pet food product that was contaminated with aflatoxin [32]. Pet food recalls by Sunshine Mills also happened in 2020, due to aflatoxin contamination from corn that was used as ingredient in the pet food [33]. Currently the FDA has an established action level of 20ppb for aflatoxin in pet foods. Many of the recalled products contained >400ppb aflatoxin. Thus, there is a need to control or reduce toxigenic A. flavus contamination.
Mite infestation in semi-moist foods
Mites are common pests of stored cereals and oilseed. Storage mites belonging to the Glycyphagidae and Acaridae families including Tyrophagus, Acarus, Lepidoglyphus and Glyciphagus are considered to be important pests in stored foods and a source of environmental allergens for humans and pets like dogs with atopic dermatitis [34,35]. These mites are often found in stored hay, straw, grain, and dry feed stuffs. In the UK, surveys have detected their presence in over 80% of stores, with Acarus siro, Lepidoglyphus destructor, Tyrophagus longior, and Tyrophagus putrescentiae in order of predominance [36,37]. Surveys from other parts of the world have found L. destructor, T. putrescentiae and Tyrophagus spp. to be the predominant species, with A. siro occurring less frequently than in temperate regions [38-40].
Among astigmatid mites, Tyrophagus putrescentiae is a cosmopolitan species commonly encountered infesting a large number of semi-moist and low moisture foods including food grains and stored food with a high fat and protein content, such as dried eggs, dried bananas, cheeses, ham, fishmeal, wheat spillage, oats, flour, and different kinds of nuts [41,42]. It causes serious economic losses as well as reducing nutrient content and germination ability [43]. In addition, the mite is responsible for allergic diseases among farmers and food industry workers handling heavily infested stored products [41] which can cause acute enteritis and systemic anaphylaxis [44] when contaminated food is ingested. The mite also acts as a carrier of bacteria and toxigenic fungi such as Aspergillus spp. in stored grain kept under warm and moist conditions [45]. When food products, including pet food, are stored at homes by consumers, they are susceptible to mite infestation from house dusts which can harbor some storage mites. Brazis et al. [46] observed that two out of ten different brands of sealed commercial dog foods contained storage mites, and upon storage at optimal temperatures and humidity, nine out of ten products contained storage mites. T. putrescentiae is a small mite species (0.28- 0.41mm) with a translucent body and virtually colorless chelicerae and limbs. The mite’s life cycle requires 2-3 weeks under ideal environmental conditions (23 °C and a relative humidity of 87%). According to Bahrami et al. [47], typical development times for eggs, larvae, and the two nymphal stages are 2, 3 and 5 days, respectively, at a temperature of 25 °C and 70%RH. There are different reports on longevity of adult mites depending on temperature, relative humidity, and food source [48]. The lifespan of the mites reared on wheat germ at 25 °C and 85% RH was about 60 days. In terms of food impact on longevity, it was up to 120 days for mites reared on wheat germ, 80 days on pumpkin seeds, 75 days on powdered milk and 25 days on rolled oats. A female mite reared on wheat germ or yeast can produce 500 eggs [48].
Contaminants from food contact surfaces
Although the water activity range of semi-moist foods are mostly unfavorable for the growth of pathogenic bacteria, contaminated food contact surfaces can transmit and perpetuate pathogens to foods including semi-moist foods in industrial and domestic food handling environments, thereby creating food safety issues through cross contamination. Exposure to pathogens on surfaces may take place either directly by contact with contaminated objects or indirectly via aerosols originating from the surface. Various bacteria of public health significance, including Salmonella, E. coli, and Listeria, can survive on hands, sponges, clothes, utensils, and currency for hours or days [49]. Pathogenic bacteria may remain on equipment surfaces even after disinfection procedures are applied, increasing the risks associated with the transmission of diseases [50]. Not only bacteria, but mold spores from the environment may also remain on equipment surfaces, on the floor of food manufacturing facilities, on automobile tires etc. and eventually find their way to contaminate foods. Therefore, domestic and industrial food handling environments can be important sources of foodborne pathogens and spoilage organisms including bacteria and molds. Further complicating biological hazard control on these surfaces is the potential formation of biofilms. Biofilms are formed when individual bacterial cells adhere and embed in an extracellular polymeric substance on surfaces such as processing equipment providing a defense mechanism [51]. Pathogenic bacteria, including Salmonella spp. and Listeria monocytogenes, may form biofilms in processing environments [51]. Their extracellular polymeric matrix is difficult to penetrate for sanitizing, for example, Salmonella spp. has been shown to maintain their presence on dry surfaces for up to 4 weeks through a biofilm [52].
Application of Clean-Label Components
According to an annual poll conducted by the Center for Food Integrity, consumers have less confidence in the safety and quality of the food supply and are demanding more all-natural and minimally processed foods with less synthetic chemical additives [53]. Consumers also have increased interest in organic foods because they are often viewed to be healthier, better tasting, or fresher than conventional products [54]. However, though free of synthetic chemicals, organic and all-natural foods are not exempt from bacterial and fungal contamination and may require the addition of antimicrobials to ensure their safety. All-natural, cleanlabel antimicrobials including those derived from plants, animals and bacteria have been shown to be effective at increasing the safety of food products by destroying or limiting the growth of pathogens and spoilage organisms [55,56].
Mold inhibitors
The most commonly used chemical preservatives to prevent mold spoilage in semi-moist foods include: (i) propionates (calcium or sodium propionate), (ii) sorbates (sorbic acid and potassium sorbate), (iii) benzoates, (iv) parabens (methyl and propyl), and (v) acetic acid [57]. Calcium and sodium propionate are the neutral salts of propionic acid. Propionate is a naturally occurring byproduct of the Propionibacterium spp. found in Swiss cheese. Due to their lack of activity against yeast, propionates are the most widely used antimicrobial and mold inhibitor in yeast-raised baked foods [57]. Propionic acid is industrially produced by the hydro-carboxylation of ethylene and the aerobic oxidation of propionaldehyde [58]. Calcium propionate and sodium propionate are produced by the acid-base reaction of calcium carbonate and sodium hydroxide with propionic acid. Sorbic acid, and its sodium and potassium salts, are effective against yeasts and molds. Consequently, since these products can inhibit yeast fermentation, sorbates are applied to semi-moist food products as encapsulates sprayed onto the product as an aerosol or incorporated into packaging material. Sorbic acid was first isolated from the berries of Mountain Ash (Sorbus spp.) trees [59] and is commercially prepared by synthetic procedures, such as the condensation of crotonaldehyde and ketene [60]. Benzoates (sodium benzoate) are inhibitory to yeast and mold and most commonly used to delay spoilage of high acid fillings, fruits and jams [57]. Sodium benzoate is manufactured by the neutralization of benzoic acid with sodium hydroxide [61]. Benzoic acid naturally occurs in cranberries, prunes and cinnamon. Parabens are related to benzoic acid-esters of para-hydroxybenzoic acid. Because they share benzoic acid’s ability to inhibit yeast and mold activity, parabens are typically used in cereal and potatobased snacks [57].
Although these are effective mold inhibitors, they are considered to be ‘synthetic’ chemical additives. In recent times, consumers have preferred natural, clean label ingredients in their foods. Thus, there has been growing research to investigate cleanlabel mold inhibiting components to improve shelf life of semimoist foods. Table 3 summarizes some of the previous research that investigated natural/clean label ingredients to inhibit mold growth in semi-moist foods [20,62-108] Table 3.
Table 3:Natural/clean label ingredients inhibit mold growth in semi-moist foods.
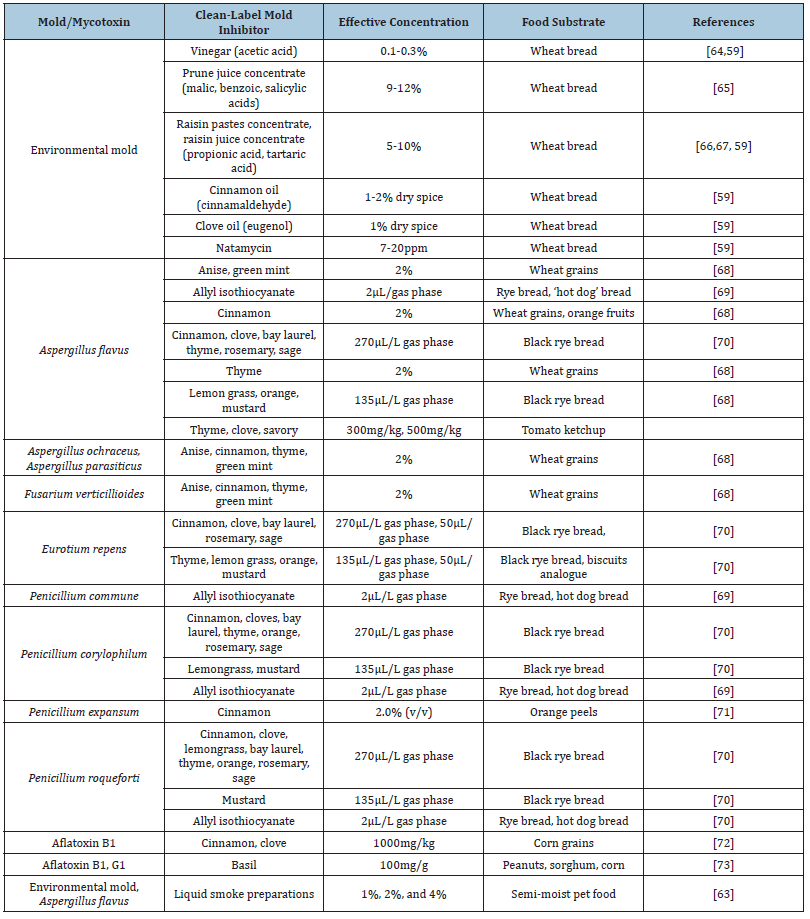
When it comes to the mode of action of natural mold inhibitors, the chemical structure of the natural plant essential oil components and their antifungal properties are found to be related. The presence and position of the hydroxyl group in the molecule, the presence of the aromatic ring, solubility in fats and spatial orientation affect the antifungal activity [74,75]. Compounds containing aromatic rings include phenolic compounds and are characterized by high antimicrobial activity [76,77]. Also, the presence of an alkyl group on the benzene ring of phenols or guaiacol [78], acetate groups (for example, geranyl-acetate has a stronger antimicrobial activity than geraniol, and bornyl-acetate than borneol) [79], oxygen in monoterpenes and their carbonylated compounds [80] enhance the antifungal activity of the components. Phenolic components (carvacrol, thymol, eugenol etc.) exhibit the strongest antifungal and antimycotoxigenic activity, followed by alcohols, aldehydes, ketones, esters and hydrocarbons [81]. A possible mechanism of action for plant essential components on the growth of fungi has been reported in several studies. It is generally accepted that the essential oil components act on the functionality and the structure of the cell membrane [82]. Low concentrations result in changes in the cell’s structure, inhibiting respiration and changing the permeability of the cell membrane, whereas high concentrations lead to severe membrane damage, loss of homeostasis and cell death [83]. Conner et al. [84] suggested that the antifungal activity is the product of essential oil components’ interaction with enzymes responsible for energy production and the synthesis of structural compounds of the cell. On the other hand, Omidbeygi et al. [85] suggested that the essential oil components pass through the cell membrane, integrate with membrane enzymes and proteins of membranes, causing a loss of macromolecules from the interior of the cell, and thereby leading to changes in the cell and ultimately to its death.
Control of mite infestation
Traditionally, control of mite infestation in stored foods depends on chemical methods such as fumigation with methyl bromide, spraying with organophosphorus compounds, or treatment with pesticides like benzyl benzoate and repellents like DEET (N, N-diethyl-m-toluamide) because ecological control methods with high humidity and temperature cause alterations in food quality. Residual pesticides are mainly used to treat the structure of buildings, and some are applied directly to the food commodity. Methyl bromide, though an effective fumigant used to control storage mites like T. putrescentiae, has been phased out in industrialized nations across the world, including the US, due to its ozone-depleting nature. Numerous efficacy tests have been performed with experimental and formulated acaricides against mold mites in the context of agricultural and industrial settings [86-90]. They leave behind some residue on the treated commodity, and repeated use of these chemicals have resulted in the development of resistance by the mites [91]. These chemicals have had undesirable effects on non-target organisms and have led to environmental and human health concerns [92]. These problems have highlighted the need for the development of new strategies for targeted storage mite control, preferably using safe, non-toxic, and natural compounds.
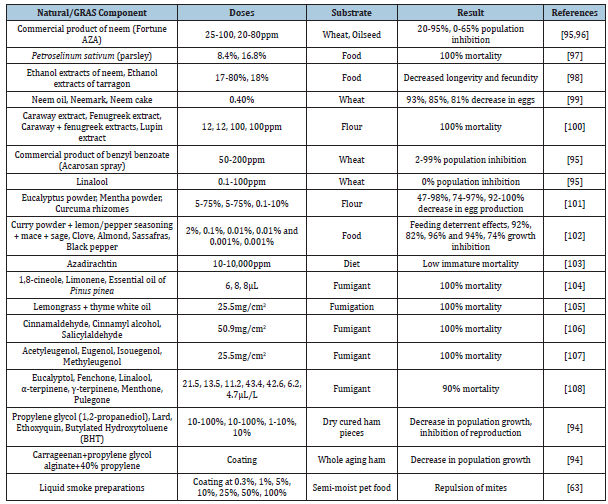
Some of the previous research studies have investigated natural/ clean label components to inhibit T. putrescentiae infestation, which are summarized in Table 4 [93,94,63].
Utilizing mitigation strategies along with good sanitation practices in the industry and storage spaces help in developing effective integrated pest management for mites in these areas.
Table 4:Natural/GRAS (Generally Recognized as Safe) ingredients evaluated in food substances to mitigate T. putrescentiae infestation.
Sanitization of food contact surfaces
Preventive measures utilized by the food industry to decontaminate and sanitize food contact surfaces have included coating of surfaces to limit the establishment of vegetative cells or biofilms [109]. Huss et al. [110] and Schumacher et al. [111] demonstrated that liquid decontamination of animal food manufacturing equipment appears effective but were not very practical or easy to implement. Generally, a water activity level >0.87aw is required for growth of most bacterial pathogens of concern including Salmonella, so introducing a water-based sanitizer would raise the aw to levels that allow for Salmonella growth. Chlorine and chlorine derivatives have been used as a broad-spectrum bactericidal, fungicidal, sporicidal, tuberculocidal, and virucidal control within food manufacturing facilities. Hypochlorite and sodium chlorite can be effective detergents and sanitizers and are known to penetrate biofilms developed by Salmonella [112,113]. However, hypochlorite solutions may produce the carcinogens bischloromethyl ether when in contact with formaldehyde [114], and trihalomethane when in contact with hot water. Because of their potential impact on human health, chlorine and its derivatives must be rinsed from surfaces prior to manufacturing food for consumption by humans or pet animals. Quaternary ammonium compounds are known to be effective sanitizers of fungi, bacteria, and non-enveloped viruses. Their true advantage is their ability to effectively sanitize Salmonella spp. biofilms and because the presence of organic matter is not as inhibitory to their action as it is to other sanitizers [115]. These compounds have been demonstrated to reduce Salmonella spp. by 2 to 3 CFU/cm2 log on galvanized steel [113]. Research has also demonstrated their effectiveness to sanitize stainless steel contaminated with Listeria [116,117] and rubber contaminated with Salmonella [117].
Due to their effectiveness and positive consumer perception of being chemical-free and non-toxic, acid-anionic clean-label sanitizers like acetic, benzoic, and propionic acids have been favored as antimicrobials in food processing facilities. Their mode of action includes acidifying the cytoplasm and disrupting cell membrane organization, which is highly effective for Salmonella destruction in food manufacturing facilities [118,119]. Due to their effectiveness and relative safety, organic acid salts have also been used as surface treatment of foods to prevent microbial growth and as a preservative in finished foods [120,121]. For example, sodium bisulfate, a dry GRAS acidulant, has been demonstrated to prevent Salmonella cross-contamination when used as a coating on pet food kibbles [122] and reduce Shiga Toxin-Producing Escherichia Coli (STEC) loads in wheat during tempering [123]. Recent research has utilized antimicrobial properties of medium chain fatty acids for reduction of Salmonella Typhimurium in animal feed [124] and pet food [125]. Other fatty acids have been used for inclusion in poultry diets for salmonellosis control. Believed antimicrobial modes of action include disruption of the cell membrane and other essential functions [126]. Cochrane [124] demonstrated efficacy of medium chain fatty acids on the reduction of porcine epidemic diarrhea virus. Deliephan et al. [127] demonstrated efficacy of organic acid mixtures containing hydroxy-4-methylthio-butanoic acid (HMTBa) on the reduction of Salmonella on food contact surfaces, and on the reduction of Salmonella and STEC when used as a coating on pet food kibbles [128]. Similarly, 3-hydroxy-3-methylbutyric acid (HMB) was also shown to mitigate Salmonella when coated on pet food kibbles [129]. There has been growing interest in investigating novel food-safe sanitizers to decontaminate food contact surfaces in manufacturing facilities.
Conclusion
This paper reviewed the important food safety issues that affect semi-moist or intermediate moisture foods in the human food and pet food industry, and the application of natural clean-label components in mitigating them. Consumers are increasingly aware of the risks associated with the use of synthetically manufactured additives and preservatives in the food industry. Clean label ingredients have been found to be effective in inhibiting pathogen and spoilage organisms; however, some gaps exist in optimization of dosage levels in foods without compromising on sensory attributes. More systematic research is required to further explore and fully utilize the potential of clean label ingredients for enhancing food safety and consumer acceptance of semi-moist foods.
Acknowledgement
This paper is contribution number 23-207-J of the Kansas Agricultural Experiment Station.
References
- Qiu L, Zhang M, Tang J, Adhikari B, Cao P (2019) Innovative technologies for producing and preserving intermediate moisture foods: A review. Food Research International 116: 90-102.
- United States Food and Drug Administration (US-FDA) (2016) Hazard analysis and risk-based preventive controls for human food: Guidance for industry. Center for Food Safety and Applied Nutrition. US FDA, Department of Health and Human Services, Washington, DC, USA.
- Barbosa-Cánovas GV, Fontana AJ, Schmidt SJ, Labuza TP (2020) Water activity in foods: Fundamentals and applications. In: Barbosa-Cánovas GV, et al. (Eds.), John Wiley & Sons, Hoboken, NJ, USA.
- Fellows PJ (2009) Food processing technology: Principles and practice, Woodhead publishing, Cambridge, England.
- Severini C, Corbo MR, Derossi A, Bevilacqua A, Giuliani R (2008) Use of humectants for the stabilization of pesto sauce. International Journal of Food Science & Technology 43(6) 1041-1046.
- Zicker SC (2008) Evaluating pet foods: how confident are you when you recommend a commercial pet food? Topics in Companion Animal Medicine 23(3): 121-126.
- Beuchat LR (1981) Microbial stability as affected by water activity. Cereal Foods World 26(7): 345-349.
- Leistner L, Gould GW (2012) Hurdle technologies: Combination treatments for food stability, safety and quality. Springer Science & Business Media, New York, United States.
- Roberts TA, Smart JL (1976) The occurrence and growth of Clostridium spp. in vacuum‐packed bacon with particular reference to perfringens (welchii) and C. botulinum. International Journal of Food Science & Technology 11(3): 229-244.
- Jakobsen M (1985) Effect of aw on growth and survival of Bacillaceae. In: Jakobsen M (Ed.), Properties of Water in Foods, Springer, Dordrecht, Netherlands, pp. 259-272.
- Corry JE (1976) Sugar and polyol permeability of Salmonella and osmophilic yeast cell membranes measured by turbidimetry, and its relation to heat resistance. Journal of Applied Bacteriology 40(3): 277-284.
- Cole MB, Jones MV, Holyoak C (1990) The effect of pH, salt concentration and temperature on the survival and growth of Listeria monocytogenes. Journal of Applied Bacteriology 69(1): 63-72.
- De Daza MT, Villegas Y, Martinez A (1991) Minimal water activity for growth of Listeria monocytogenes as affected by solute and temperature. International Journal of Food Microbiology 14(3-4): 333-337.
- Vogel P, Dal Bosco SM, Ferla NJ (2015) Mites and the implications on human health. Nutricion Hospitalaria 31(2): 944-951.
- Wisniewski H, Sigurdarson S, Rubenstein R, Kascsak R, Carp R (1996) Mites as vectors for scrapie. The Lancet 347(9008): 1114.
- Sinha RN, Mills JT (1968) Feeding and reproduction of grain mite and the mushroom mite on some species of Penicillium. Journal of Economic Entomology 61(6): 1548-1552.
- Anthony J, Fontana J (2007) Minimum water activity limits for growth of microorganisms. In: Gustavo VB, et al. (Eds.), Water activity in foods: Fundamentals and applications, Blackwell Publishing, Hoboken, NJ, USA, p. 405.
- Samson RA, Hoekstra ES, Frisvad JC (2004) Introduction to food-and airborne fungi. (7th edn), Centraal bureau voor Schimmelcultures (CBS), CAB Direct, Utrecht, Netherlands, p.389.
- Pitt JI, Hocking AD (2009) Fungi and food spoilage, Springer, New York, NY, United States, pp. 388.
- Kocić-Tanackov SD, Dimić GR (2013) Antifungal activity of essential oils in the control of food-borne fungi growth and mycotoxin biosynthesis in food. Metabolism, 4.
- Janik E, Niemcewicz M, Ceremuga M, Stela M, Saluk-Bijak J, et al. (2020) Molecular aspects of mycotoxins-A serious problem for human health. International Journal of Molecular Sciences 21(21): 8187.
- Wang JS, Groopman JD (1999) DNA damage by mycotoxins. Mutation Research/Fundamental and Molecular Mechanisms of Mutagenesis 424(1-2): 167-181.
- Diaz DE (2005) The Mycotoxin Blue Book. Nottingham University Press, Nottingham, England.
- Bueno DJ, Silva JO, Oliver G (2001) Mycoflora in commercial pet foods. Journal of Food Protection 64(5): 741-743.
- Martins ML, Martins HM, Bernardo F (2003) Fungal flora and mycotoxins detection in commercial pet food. Revista Portuguesa de Ciências Veterinárias 98(548): 179-183.
- Scudamore KA, Hetmanski MT, Nawaz S, Naylor J, Rainbird S (1997) Determination of mycotoxins in pet foods sold for domestic pets and wild birds using linked‐column immunoassay clean‐up and HPLC. Food Additives & Contaminants 14(2): 175-186.
- Savi GD, Piacentini KC, Scussel VM (2015) Ozone treatment efficiency in Aspergillus and Penicillium growth inhibition and mycotoxin degradation of stored wheat grains (Triticum aestivum). Journal of Food Processing and Preservation 39(6): 940-948.
- Moss MO (1996) Mycotoxic fungi. In: Eley AR (Ed.), Microbial Food Poisoning. (2nd edn), Chapman and Hall, New York, NY, USA, pp. 75-93.
- International Agency for Research on Cancer-World Health Organization (IARC-WHO) (1993) Some naturally occurring substances: Food items and constituents, heterocyclic aromatic amines and mycotoxins. In: IARC Monographs on the Evaluation of Carcinogenic Risks to Humans; IARC Working Group, World Health Organization: Lyon, France, 56: 467-488.
- Silva J, Pereira MN, Scussel VM (2018) Ozone gas antifungal effect on extruded dog food contaminated with Aspergillus flavus. Ozone: Science & Engineering 40(6): 487-493.
- Leung MC, Díaz-Llano G, Smith TK (2006) Mycotoxins in pet food: a review on worldwide prevalence and preventative strategies. Journal of Agricultural and Food Chemistry 54(26): 9623-9635.
- United States-Food and Drug Administration (US-FDA) (2020a) FDA alert: Certain lots of Sportmix pet food recalled for potentially fatal levels of aflatoxin.
- United States-Food and Drug Administration (US-FDA) (2020b) Sunshine Mills, Inc. issues voluntary recall of certain products due to potentially elevated levels of aflatoxin.
- Vollset I, Larsen HJ, Mehl R (1986) Immediate type hypersensitivity in dogs induced by storage mites. Research in Veterinary Science 40(1): 123-127.
- Nuttall TJ, Hill PB, Bensignor E, Willemse T, members of the International Task Force on Canine Atopic Dermatitis (2006) House dust and forage mite allergens and their role in human and canine atopic dermatitis. Veterinary Dermatology 17(4): 223-235.
- Lynch NR, Thomas WR, Chua Y, García N, Di Prisco MC, et al. (1994) In vivo biological activity of recombinant Der p II allergen of house-dust mite. International Archives of Allergy and Immunology 105(1): 70-74.
- Prickett AJ (1997) Oilseed Stores 1995, England, Pest Management. Ministry of Agriculture, Fisheries and Food Central Science Laboratory, Sand Hutton, England, p.62.
- Mahmood SH (1992) Mite fauna of stored grain seeds in central Iraq. Journal of Stored Products Research 28(3): 179-181.
- Emmanouel NG, Buchelos CT, Dukidis CTE (1994) A survey on the mites of stored grain in Greece. Journal of Stored Products Research 30(2): 175-178.
- Haines CP (1997) Insects and arachnids in Indonesian food stores - Biodiversity in a man-made environment. Proceedings of the Symposium on Pest Management for Stored Food and Feed, BIOTROP Special Publication, Bogor, Indonesia, 59: 95-125,
- Hughes AM (1976) The mites of stored food and houses. (2nd edn), Her Majesty's Stationery Office, London, UK.
- Sinha RN (1979) Role of Acarina in the stored grain ecosystem. Recent Advances in Acarology 1: 263-272.
- Krantz GW (1955) Some mites injurious to farm-stored grain. Journal of Economic Entomology 48(6): 754-755.
- Matsumoto T, Hisano T, Hamaguchi M, Miike T (1996) Systemic anaphylaxis after eating storage-mite-contaminated food. International Archives of Allergy and Immunology 109(2): 197-200.
- Franzolin MR, Gambale W, Cuero RG, Correa B (1999) Interaction between toxigenic Aspergillus flavus Link and mites (Tyrophagus putrescentiae Schrank) on maize grains: effects on fungal growth and aflatoxin production. Journal of Stored Products Research 35(3): 215-224.
- Brazis P, Serra M, Sellés A, Dethioux F, Biourge V, et al. (2008) Evaluation of storage mite contamination of commercial dry dog food. Veterinary Dermatology 19(4): 209-214.
- Bahrami F, Kamali K, Fathipour Y (2007) Life history and population growth parameters of Tyrophagus putrescentiae (Acari: Acaridae) on Fusarium graminearum in laboratory conditions. Journal of Entomological Society of Iran 26: 7-18.
- Boczek J (1991) Mite pests in stored food. In: Richard GJ (Ed.), Ecology and management of food-industry pests, Association of Official Analytical Chemist, Arlington, VA, USA, pp. 57-79.
- Scott E, Bloomfield SF (1990) The survival and transfer of microbial contamination via cloths, hands and utensils. Journal of Applied Bacteriology 68(3): 271-278.
- Dunsmore DG, Twomey A, Whittlestone WG, Morgan HW (1981) Design and performance of systems for cleaning product-contact surfaces of food equipment: A review. Journal of Food Protection 44(3): 220-240.
- McLandsborough L, Rodriguez A, Pérez-Conesa D, Weiss J (2006) Biofilms: At the interface between biophysics and microbiology. Food Biophysics 1: 94-114.
- Habimana O, Moretro T, Langsrud S, Vestby LK, Nesse LL, et al. (2010) Micro ecosystems from feed industry surfaces: A survival and biofilm study of Salmonella versus host resident flora strains. BMC Veterinary Research 6: 1-10.
- Andrews FM, Withey SB (2012) Social indicators of well-being: Americans' perceptions of life quality. Springer Science & Business Media, New York, NY, USA.
- Wier M, Calverley C (2002) Market potential for organic foods in Europe. British Food Journal 104(1): 45-62.
- Juneja VK, Dwivedi HP, Yan X (2012) Novel natural food antimicrobials. Annual Review of Food Science and Technology 3: 381-403.
- Davidson PM, Critzer FJ, Taylor TM (2013) Naturally occurring antimicrobials for minimally processed foods. Annual Review of Food Science and Technology 4: 163-190.
- Pyler EJ, Gorton LA (2008) Baking Science & Technology. (4th edn), Sosland Publishing Company, Kansas City, MO, USA, pp. 456-459.
- Samel U, Kohler W, Gamer A, Keuser U (2005) Propionic acid and derivatives. Ullmann’s Encyclopedia of Industrial Chemistry, Weinheim, Germany.
- Bullerman L (2000) Mold Growth in Bakery Products and Its Prevention. AIB Technical Bulletin 22(6).
- Weissermel K, Arpe H (2003) Industrial Organic Chemistry, 4th edition, Weinheim, Germany, p. 187.
- Wibbertmann A, Kielhorn J, Koennecker G, Mangelsdorf I, Melber C (2000) Concise International Chemical Assessment Document No. 26. Benzoic Acid and Sodium Benzoate. International Program on Chemical Safety, United Nations, Geneva, Switzerland.
- Albers-Nelson R (2010) Clean label mold inhibitors for baking. Oklahoma State University Cooperative Extension, FAPC-173, Stillwater, OK, USA.
- Deliephan A (2022) Reducing spoilage in intermediate moisture pet foods using food-safe additives as a model system. Doctoral dissertation, Kansas State University, Manhattan, KS, USA.
- Dubois D (1983) Technical Assistance Industry Problems and Replies, III. AIB Technical Bulletin. 5(1): 5.
- Sanders SW (1991) Using prune juice concentrate in whole wheat bread and other bakery products. Cereal Foods World, 36: 280-283.
- Fagrell E (1992) Raisin Usage in Baked Goods. AIB Research Department Technical Bulletin 14(4): 1-8.
- Labell F (2000) Raisins=Sweetness, Functionality and Nutrition. Prepared Foods, Module-1 AIB International Correspondence Course, Manhattan, KS, USA.
- Soliman KM, Badeaa RI (2002) Effect of oil extracted from some medicinal plants on different mycotoxigenic fungi. Food and Chemical Toxicology 40(11): 1669-1675.
- Nielsen PV, Rios R (2000) Inhibition of fungal growth on bread by volatile components from spices and herbs, and the possible application in active packaging, with special emphasis on mustard essential oil. International Journal of Food Microbiology 60(2-3): 219-229.
- Suhr KI, Nielsen PV (2003) Antifungal activity of essential oils evaluated by two different application techniques against rye bread spoilage fungi. Journal of Applied Microbiology 94(4): 665-674.
- Xing Y, Li X, Xu Q, Yun J, Lu Y (2010) Antifungal activities of cinnamon oil against Rhizopus nigricans, Aspergillus flavus and Penicillium expansum in vitro and in vivo fruit test. International Journal of Food Science & Technology 45(9): 1837-1842.
- Sinha KK, Sinha AK, Prasad G (1993) The effect of clove and cinnamon oils on growth of and aflatoxin production by Aspergillus flavus. Letters in Applied Microbiology 16(3): 114-117.
- Atanda OO, Akpan I, Oluwafemi F (2007) The potential of some spice essential oils in the control of parasiticus CFR 223 and aflatoxin production. Food Control 18(5): 601-607.
- Raccach M (1984) The antimicrobial activity of phenolic antioxidants in foods: A review. Journal of Food Safety 6(3): 141-170.
- Veluri R, Weir TL, Bais HP, Stermitz FR, Vivanco JM (2004) Phytotoxic and antimicrobial activities of catechin derivatives. Journal of Agricultural and Food Chemistry 52(5): 1077-1082.
- Farag RS, Daw ZY, Hewedi FM, El-Baroty GSA (1989) Antimicrobial activity of some Egyptian spice essential oils. Journal of Food Protection 52(9): 665-667.
- Decker EA (1995) The role of phenolics, conjugated linoleic acid, carnosine, and pyrroloquinoline quinone as nonessential dietary antioxidants. Nutrition Reviews 53(3): 49-58.
- Kurita N, Miyaji M, Kurane R, Takahara Y (1981) Antifungal activity of components of essential oils. Agricultural and Biological Chemistry 45(4): 945-952.
- Dorman HD, Deans SG (2000) Antimicrobial agents from plants: Antibacterial activity of plant volatile oils. Journal of Applied Microbiology 88(2): 308-316.
- Naigre R, Kalck P, Roques C, Roux I, Michel G (1996) Comparison of antimicrobial properties of monoterpenes and their carbonylated products. Planta Medica 62(3): 275-277.
- Kurita N, Koike S (1983) Synergistic antimicrobial effect of ethanol, sodium chloride, acetic acid and essential oil components. Agricultural and Biological Chemistry 47(1): 67-75.
- Viuda-Martos M, Ruiz-Navajas Y, Fernández-López J, Pérez-Álvarez J (2008) Antifungal activity of lemon (Citrus lemon), mandarin (Citrus reticulata L.), grapefruit (Citrus paradisi L.) and orange (Citrus sinensis L.) essential oils. Food Control 19(12): 1130-1138.
- Carson CF, Mee BJ, Riley TV (2002) Mechanism of action of Melaleuca alternifolia (tea tree) oil on Staphylococcus aureus determined by time-kill, lysis, leakage, and salt tolerance assays and electron microscopy. Antimicrobial Agents and Chemotherapy 46(6): 1914-1920.
- Conner DE, Beuchat LR (1984) Effects of essential oils from plants on growth of food spoilage yeasts. Journal of Food Science 49(2): 429-434.
- Omidbeygi M, Barzegar M, Hamidi Z, Naghdibadi H (2007) Antifungal activity of thyme, summer savory and clove essential oils against Aspergillus flavus in liquid medium and tomato paste. Food Control 18(12): 1518-1523.
- Kim EH, Kim HK, Choi DH, Ahn YJ (2003) Acaricidal activity of clove bud oil compounds against Tyrophagus putrescentiae (Acari: Acaridae). Applied Entomology and Zoology 38(2): 261-266.
- Palyvos NE, Athanassiou CG, Kavallieratos NG (2006) Acaricidal effect of a diatomaceous earth formulation against Tyrophagus putrescentiae (Astigmata: Acaridae) and its predator Cheyletus malaccensis (Prostigmata: Cheyletidae) in four grain commodities. Journal of Economic Entomology 99(1): 229-236.
- Hubert J, Stejskal V, Munzbergova Z, Hajslova J, Arthur FH (2007) Toxicity and efficacy of selected pesticides and new acaricides to stored product mites (Acari: Acaridida). Experimental and Applied Acarology 42(4): 283-290.
- Jeong EY, Lim JH, Kim HG, Lee HS (2008) Acaricidal activity of Thymus vulgaris oil and its main components against Tyrophagus putrescentiae, a stored food mite. Journal of Food Protection 71(2): 351-355.
- Stara J, Stejskal V, Nesvorná M, Plachý J, Hubert J (2011) Efficacy of selected pesticides against synanthropic mites under laboratory assay. Pest Management Science 67(4): 446-457.
- Szlendak E, Conyers C, Muggleton J, Thind BB (2000) Pirimiphos-methyl resistance in two stored product mites, Acarus siro and Acarus farris, as detected by impregnated paper bioassay and esterase activity assays. Experimental & Applied Acarology 24(1): 45-54.
- Hays JB, Laws ER (1991) Handbook of Pesticide Toxicology, Volume 1, Academic Press, Cambridge, MA, USA.
- Collins DA (2006) A review of alternatives to organophosphorus compounds for the control of storage mites. Journal of Stored Products Research 42(4): 395-426.
- Abbar S, Amoah B, Schilling MW, Phillips TW (2016) Efficacy of selected food‐safe compounds to prevent infestation of the ham mite, Tyrophagus putrescentiae (Schrank) (Acarina: Acaridae), on southern dry‐cured hams. Pest Management Science 72(8): 1604-1612.
- Collins DA, Armitage DM, Cook DA, Buckland A, Bell J (2001) The efficacy of alternative compounds to organophosphorus pesticides for the control of storage mite pests, Home-Grown Cereals Authority report no. 249, London, UK, p.163.
- Collins DA (2003) The efficacy of flufenoxuron, azadirachtin and a diatomaceous earth, when admixed with oilseed rape, against storage mite pests. In: Credland PF, Armitage DM, et al. (Eds.), Proceedings of the Eighth International Working Conference on Stored Product Protection, York, England, 22–26 July 2002. CAB International, Wallingford, Oxon, England, pp. 685-688.
- Czajkowska B (1971) The influence of some active substances of medicinal herbs on stored product mites (Acaroidea). In: Daniel MR (Ed.), Proceedings of the Third International Congress of Acarology, Prague, Czechoslovakia. Junk W, BV Publishers, The Hague and Academia, Publishing House of the Czechoslovak Academy of Sciences, Prague, Czechoslovakia, pp. 365-369.
- Czajkowska B (2002) Effect of some extracts of medicinal and spicy plants on bionomy of stored product mites. Folia Horticulturae 14(1): 201-211.
- Gulati R (1998) Inhibitory action of neem products on Tyrophagus putrescentiae (Schrank) (Acarina: Acaridae) in wheat during storage. Annals of Agri Bio Research 3: 227-230.
- Afifi FA, Hafez SM (1988) Effect of different plant extracts on the toxicity and behavior of Tyrophagus putrescentiae (Shrank) (Acari: Acaridae). Annals of Agricultural Science, Cairo, Egypt, 33: 1375-1385.
- Gulati R, Mathur S (1995) Effect of Eucalyptus and Mentha leaves and Curcuma rhizomes on Tyrophagus putrescentiae (Schrank) (Acarina: Acaridae) in wheat. Experimental and Applied Acarology 19: 511-518.
- Rodriguez JG, Potts MF, Patterson G (1979) Allelochemic effects of some flavoring components on the acarid, Tyrophagus putrescentiae. In: Rodriguez JG (Ed.), Recent Advances in Acarology, volume 1, Academic Press, Cambridge, MA, USA, pp. 251-262.
- Sanchez-Ramos I, Castanera P (2003) Laboratory evaluation of selective pesticides against the storage mite Tyrophagus putrescentiae (Acari: Acaridae). Journal of Medical Entomology 40(4): 475-481.
- Macchioni F, Cioni PL, Flamini G, Morelli I, Perrucci S, et al. (2002) Acaricidal activity of pine essential oils and their main components against Tyrophagus putrescentiae, a stored food mite. Journal of agricultural and food chemistry 50(16): 4586-4588.
- Kim EH, Kim HK, Ahn YJ (2003) Acaricidal activity of plant essential oils against Tyrophagus putrescentiae (Acari: Acaridae). Journal of Asia-Pacific Entomology 6(1): 77-82.
- Kim HK, Kim JR, Ahn YJ (2004) Acaricidal activity of cinnamaldehyde and its congeners against Tyrophagus putrescentiae (Acari: Acaridae). Journal of Stored Products Research 40(1): 55-63.
- Kim EH, Kim HK, Choi DH, Ahn YJ (2003) Acaricidal activity of clove bud oil compounds against Tyrophagus putrescentiae (Acari: Acaridae). Applied Entomology and Zoology 38(2): 261-266.
- Sanchez-Ramos I, Castanera P (2001) Development and survival of Tyrophagus putrescentiae (Acari: Acaridae) at constant temperatures. Environmental Entomology 30(6): 1082-1089.
- Jullien C, Bénézech T, Carpentier B, Lebret V, Faille C (2003) Identification of surface characteristics relevant to the hygienic status of stainless steel for the food industry. Journal of Food Engineering 56(1): 77-87.
- Huss AR, Cochrane RA, Deliephan A, Stark CR, Jones CK (2015) Evaluation of a biological pathogen decontamination protocol for animal feed mills. Journal of Food Protection 78(9): 1682-1688.
- Schumacher LL, Cochrane RA, Evans CE, Kalivoda JR, Woodworth JC, et al. (2016) Evaluating the effect of manufacturing Porcine Epidemic Diarrhea Virus (PEDV)-contaminated feed on subsequent feed mill environmental surface contamination. Journal of Animal Science 94: 77-77.
- Joseph B, Otta SK, Karunasagar I, Karunasagar I (2001) Biofilm formation by Salmonella spp. on food contact surfaces and their sensitivity to sanitizers. International Journal of Food Microbiology 64(3): 367-372.
- Ramesh N, Joseph SW, Carr LE, Douglass LW, Wheaton FW (2002) Evaluation of chemical disinfectants for the elimination of Salmonella biofilms from poultry transport containers. Poultry Science 81(6): 904-910.
- Gamble MR (1977) Hazard: Formaldehyde and hypochlorites. Laboratory Animals 11(1): 61.
- Betty RG, Bieker JM, Tucker MD (2005) Agricultural pathogen decontamination technology: Reducing the threat of infectious agent spread. No. SAND2006-0182, Sandia National Laboratories, Albuquerque, NM, United States.
- Pan Y, Breidt F, Kathariou S (2006) Resistance of Listeria monocytogenes biofilms to sanitizing agents in a simulated food processing environment. Applied and Environmental Microbiology 72(12): 7711-7717.
- Ronner AB, Wong AC (1993) Biofilm development and sanitizer inactivation of Listeria monocytogenes and Salmonella typhimurium on stainless steel and Buna-n rubber. Journal of Food Protection 56(9): 750-758.
- Ricke SC (2003) Perspectives on the use of organic acids and short chain fatty acids as antimicrobials. Poultry Science 82(4): 632-639.
- Carrique‐Mas JJ, Bedford S, Davies RH (2007) Organic acid and formaldehyde treatment of animal feeds to control Salmonella: Efficacy and masking during culture. Journal of Applied Microbiology 103(1): 88-96.
- Smulders FJM (1995) Preservation by microbial decontamination; the surface treatment of meats by organic acids. In New methods of food preservation. Springer, Boston, MA, United States, pp. 253-282.
- Samelis J, Bedie GK, Sofos JN, Belk KE, Scanga JA, et al. (2005) Combinations of nisin with organic acids or salts to control Listeria monocytogenes on sliced pork bologna stored at 4 C in vacuum packages. LWT-Food Science and Technology 38(1): 21-28.
- Jeffrey A (2016) The role of Salmonella in animal food. M.S. Thesis, Kansas State University, Manhattan, Kansas, USA.
- Rivera J, Deliephan A, Dhakal J, Aldrich CG, Siliveru K (2021) Significance of Sodium Bisulfate (SBS) tempering in reducing) the Escherichia coli O121 and O26 load of wheat and its effects on wheat flour quality. Foods 10(7): 1479.
- Cochrane RA, Huss AR, Aldrich GC, Stark CR, Jones CK (2016) Evaluating chemical mitigation of Salmonella Typhimurium ATCC 14028 in animal feed ingredients. Journal of Food Protection 79(4): 672-676.
- Dhakal J, Aldrich CG (2020) Use of medium chain fatty acids to mitigate Salmonella typhimurium (ATCC 14028) on dry pet food kibbles. Journal of Food Protection 83(9): 1505-1511.
- Desbois AP, Smith VJ (2010) Antibacterial free fatty acids: activities, mechanisms of action and biotechnological potential. Applied Microbiology and Biotechnology 85(6): 1629-1642.
- Deliephan A, Dhakal J, Subramanyam B, Aldrich CG (2023) Mitigation of Salmonella on food contact surfaces by using organic acid mixtures containing 2-hydroxy-4-(methylthio) butanoic acid (HMTBa). Foods 12(4): 874.
- Deliephan A, Dhakal J, Subramanyam B, Aldrich CG (2023) Use of organic acid mixtures containing 2-hydroxy-4-(methylthio) butanoic acid (HMTBa) to mitigate Salmonella enterica, Shiga Toxin-producing Escherichia coli (STEC) and Aspergillus flavus in pet food kibbles. Animals 13(5): 877.
- Huss AR, Fuller JC, Centrella W, Marshall DL, Deliephan A, et al. (2017) Mitigation of Salmonella on pet food kibbles by using liquid and powdered 3-hydroxy-3-methylbutyric acid. Journal of Food Protection 80(7): 1080-1084.
© 2023 Bhadriraju Subramanyam. This is an open access article distributed under the terms of the Creative Commons Attribution License , which permits unrestricted use, distribution, and build upon your work non-commercially.
 a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in
a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in 







.jpg)






























 Editorial Board Registrations
Editorial Board Registrations Submit your Article
Submit your Article Refer a Friend
Refer a Friend Advertise With Us
Advertise With Us
.jpg)






.jpg)














.bmp)
.jpg)
.png)
.jpg)










.jpg)






.png)

.png)



.png)






