- Submissions

Full Text
Modern Concepts & Developments in Agronomy
Pumpkin Seeds into Nanoparticles
Amie E Norton1, Jeff Whitworth1* Robert Ewing2 and Lee W Cohnstaedt2
1Department of Entomology, USA
2National Bio and Agro-Defense Facility, USA
*Corresponding author: Jeff Whitworth, Department of Entomology, Manhattan, Ks, USA
Submission: October 23, 2022;Published: December 14, 2022

ISSN 2637-7659Volume12 Issue 2
Abstract
Every year 590 million kilograms of pumpkins are disposed of in landfills as organic waste. Organic waste makes-up approximately 34% of landfill mass. In order to reduce organic waste going to landfills, an alternative use of the waste or upcycling pumpkins into useful nanoparticles is described. Using a green synthesis method, gold and silver nanoparticles were synthesized..
Keywords: Green chemistry; Nanoparticles; Upcycling
Experience Report
Pumpkin is a seasonal fruit harvested from October to January in the United States of America. While approximately 2,800 million kilograms of pumpkins are produced [1], each year 590 million kilograms of those pumpkins end up in landfills as organic waste [2]. The EPA estimates that 34% of waste that ends up in landfills is organic waste [3]. Organic waste is characterized by the following products: packaging materials, animal and plant-based materials, food scraps, newspapers, cardboard, and other paper-based material. To reduce landfill use, upcycling of organic waste is required [4]. Upcycling is defined as the creation of higher value materials from objects that have been [5].
In this paper, a method is described to upcycle pumpkin seeds into gold and silver nanoparticles. Nanoscience is the study of materials at molecular and macromolecular dimensions, where the properties of the material at the nano-size differ considerably from those at a large scale [6]. Metal nanomaterials have received significant attention because the bulk material properties differ from those of the nanomaterial, making them useful in a wide array of applications. Metal nanomaterials are employed in the following applications: anticancer, radiotherapy enhancement, drug delivery, thermal ablation, antibacterial, diagnostic assays, antifungal, and gene delivery [7]. More specifically, in the agriculture sector, nanotechnology is used in the following applications: nano biosensors, plant growth regulators, plant growth promoters, nanofertilizers, nanopesticides, nutrient management, and protection against phytopathogens [8]. Two metallic nanoparticles that have received significant attention are gold and silver. Gold and silver nanoparticles are known for their unique photophysical properties as well as their antimicrobial properties [9,10].
Nanomaterials are produced from either a top-down approach or a bottom-up approach [11]. The top-down approach requires the breaking down of bulk materials into nano sizes. However, the top-down approach has many disadvantages, such as the requirement for a larger space, requires high energy, and is time consuming [11]. A more widely used approach is the bottom-up approach, which requires chemical treatments for the generation of nanoparticles [12]. However, the bottom-up approach has the disadvantage of using chemicals in the synthesis that are toxic and can lead to the generation of harmful by-products [13]. This is of particular concern when scaling up the production of nanoparticles. The solvents used in the synthesis of nanoparticles generate non-ecofriendly and sometimes toxic waste [14]. This creates a demand for “green” methods to synthesize nanomaterials. Green synthesis is defined as an environmentally friendly approach to synthesize a product by the elimination of pollution waste, decrease in energy consumption, and the use of environmental-friendly solvents (water, ethanol, etc.) [15]. One approach that has received increasing attention is the use of biosynthesis [16]. Biological synthesis involves the use of plants, bacteria, and fungi as bio-factories to produce metal nanoparticles. The use of plant extracts has been shown to be stable and durable. Plant waste can be an excellent source of various biomolecules [16].
In this paper, we describe a green method for the synthesis of gold and silver nanomaterials. This method uses water as the solvent. The method uses a plant extract synthesized from pumpkin seeds during the synthesis of the nanoparticles. This method is an example of how pumpkin seeds or plant waste can be upcycled into nanomaterials. Pumpkins were selected because 50% of the pumpkins grown are wasted each year. Gold and silver particles were selected because of the antimicrobial properties of those metals.
Materials and Methods
A fresh orange pumpkin (Cucurbita maxima) was obtained. Gold (III) chloride hydrate and silver nitrate were purchase from Sigma Aldrich (St. Louis, MO).
Preparation of Pumpkin extract/
A pumpkin was cleaned out and the seeds separated from the pulp. Fifty grams of seeds were dried using a food dehydrator (Elite gourmet, West Chester, PA) for 4 hours at 21 °C. Twenty-five grams of the dried pumpkin seeds were placed in 100mL of distilled water, heated and stirred at 32 °C for 30 minutes. The extract solution was filtered by gravity through a funnel using coarse 11cm Fisher brand filter paper (filters 2.7μm particles). This solution was stored at 4.5 °C until its use.
Synthesis of silver and gold nanoparticles
Gold and silver nanoparticles were prepared similar to literature procedures with some modifications [17,18]. In brief, 3mL of pumpkin extract solution was added to a solution of 50mL of gold or silver (5x10-5M) in distilled water. The solution was heated to 26.6 °C and stirred for 30 minutes
Characterization of nanoparticles
Nanoparticles were characterized by a Nanodrop UV-Visible spectrometer. The size of the nanoparticles was characterized by a Zetaview Quatt (Particle matrix, Ammersee, Germany) at 24 °C, using a 488nm laser.
Results
The pumpkin extract solution was used to reduce silver and gold in separate reactions. After the reaction with the extract solution, the nanoparticle solutions were characterized. One characteristic change in the reaction was the color change of the solutions. The solution of silver nitrate changed from colorless to brown, this is indicative of formation of silver nanoparticles [10,17]. The solutio of gold chloride changed from yellow to purple which is indicative of the formation of gold nanoparticles [9,18] (Figure 1).
Figure 1:Photos of solutions. 1. Pumpkin extract. 2. Silver nitrate solution. 3. Gold (III) chloride hydrate solution. 4. Solution after the reaction of pumpkin extract + Gold (III) chloride. 5. Solution after the reaction of pumpkin extract +silver nitrate.

To further characterize the nanoparticles, the UV-visible of the solutions were recorded. The color observed from gold and silver nanoparticles results from light absorption and scattering. The localized Surface Plasma Resonance Spectrum (LSPRS) is dependent on size and shape of the particles. The silver nanoparticle solution showed a new band at 425nm. A rapid method uses the wavelength maximum to determine nanoparticle size of the nanoparticles, and with this method, the size was approximately 40nm [19]. This method takes advantage that the optical properties of silver nanoparticles are affected by the size of the particles. The method calculates the change molar extinction coefficient as it correlates to particle size. Wavelength maximum and molar extinction coefficient could then be model vs. particle size. The gold nanoparticles solution showed a new band at 550nm, and a similar measuring method determined the size of the gold nanoparticles was used to calculate the particles at 65nm [20] (Figure 2).
Figure 2:UV-visible spectra of Silver Nitrate (---), Gold (III) Chloride hydrate (---), Silver Nanoparticles (___), and Gold Nanoparticles (___) in solution. In the set graph is a zoom-in region of the UV-visible spectrum from 350-750nm.
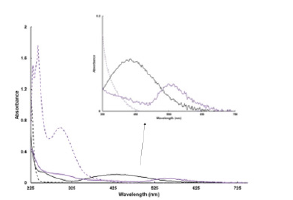
The particles size was alsomeasured using a Zetaview, which utilizes nano tracking. The mean size of the particles in solution was 167.2nm and 281nm for silver and gold, respectively. The concentration of the particles in solution was 1.2x107 particles/ ml and 6.6x107 particles/mL for silver and gold, respectively. The size determined by the UV-visible vs using the method of nano tracking are different because the Zetaview instrument takes the solvent around the particles into account [21]. The nano-tracking measures the dynamic radius of the particles, it is not related to the reflective index. The UV-visible spectrum measures the size of a particle by the optical properties of the nanoparticles. The UVvisible spectrum is sizing the plasmonic nanoparticle in the media, thus the size resulting from the UV-visible is based on the metallic part of the particle.
Conclusion
The characterization of the nanoparticles by Brownian motion and UV-visible spectroscopy demonstrated successful synthesis of gold and silver nanoparticles using pumpkin seed extracts.
References
- Hien, Tran Thi, and Nguyen Thi Minh (2021) Enhancing the extraction of pumpkin seed (Cucurbita Pepo L) for increasing oil yield and its phytosterol content. Food Science and Applied Biotechnology 4(1): 6-13.
- Ruiz A (2022) The problem with pumpkin waste. Waste Advantage Magazine (blog).
- (2017) How much waste is wasted in landfills? Ag Recycle (blog).
- Sung K (2015) A review on upcycling: current body of literature, knowledge gaps and a way forward. 17th International Conference on environmental, cultural, economic and social sustainability, Venice, Italy, pp.13-14.
- (2022) Oxford Languages. The Home of Language Data.
- Joachim C (2005) To be nano or not to be nano? Nature Materials 4(2): 107-109.
- Yaqoob AA, Hilal A, Tabassum P, Akil A, Mohammad Oves, et al. (2020) Recent advances in metal decorated nanomaterials and their various biological applications: A Review. Frontiers in Chemistry 8: 341.
- Hazarika A, Meera Y, Dinesh KY, Hardeo Singh (2022) An overview of the role of nanoparticles in sustainable agriculture. Biocatalysis and Agricultural Biotechnology 43: 102399.
- Rotimi L, Mike OO, Omobola OO, Sadimenko A, Anthony IO (2019) Synthesis, characterization, antimalarial, antitrypanocidal and antimicrobial properties of gold nanoparticle. Green Chemistry Letters and Reviews 12(1): 61-68.
- Khane Y, Khedidja B, Salim A, Ghassan MS, Mosleh MA, et al. (2022) Green synthesis of silver nanoparticles using aqueous Citrus Limon zest extract: Characterization and evaluation of their antioxidant and antimicrobial properties. Nanomaterials 12(12): 2013.
- Iqbal P, Preece JA, Mendes PM (2012) Nanotechnology: The ‘top-down’ and ‘bottom-up’ approaches. In Supramolecular Chemistry, John Wiley & Sons Ltd, USA.
- Betke A, Kickelbick G (2014) Bottom-up, wet chemical technique for the continuous synthesis of inorganic nanoparticles. Inorganics 2(1): 1-15.
- Abid N, Aqib Muhammad Khan, Shujait S, Kainat C, Muhammad Ikram, et al. (2022) Synthesis of nanomaterials using various top-down and bottom-up approaches, influencing factors, advantages, and disadvantages: A review. Advances in Colloid and Interface Science 300: 102597.
- Flegler A, Susanne W, Schneider M, Gellermann C, Mandel K (2018) Chapter 7 - tailored nanoparticles by wet chemical particle technology: From lab to pilot scale. Handbook of Nanomaterials for Industrial Applications, Micro and Nano Technologies, Elsevier, Netherlands, pp. 37-50.
- US EPA, OCSPP (2013) Basics of green chemistry. Overviews and Factsheets.
- Chums-ard W, Fawcett D, Chun Che Fung, Gerrard Eddy (2019) Biogenic synthesis of gold nanoparticles from waste watermelon and their antibacterial activity against Escherichia Coli and Staphylococcus Epidermidis. International Journal of Research in Medical Sciences 7(7): 2499.
- Ali Ban (2020) Eco-friendly synthesis of silver nanoparticles from crust of Cucurbita Maxima (red pumpkin). EurAsian Journal of BioSciences 14(2): 2829-2833.
- Iyer RI, Tapobrata P (2018) Biosynthesis of gold and silver nanoparticles using extracts of callus cultures of pumpkin (Cucurbita Maxima). Journal of Nanoscience and Nanotechnology 18(8): 5341-5353.
- Paramelle D, Sadovoy A, Gorelik S, Paul F, Jonathan H, et al. (2014) A rapid method to estimate the concentration of citrate capped silver nanoparticles from UV-visible light spectra. Analyst 139(19): 4855-4861.
- PK Jain, KS Lee, Ivan H El-Sayed, Mostafa AEl-Sayed (2006) Calculated absorption and scattering properties of gold nanoparticles of different size, shape, and composition: Applications in biological imaging and biomedicine. The Journal of Physical Chemistry B 110(14): 7238-7348.
- Tomaszewska E, Katarzyna S, Kinga K, Tkacz-Szczesna B, Celichowski G, et al. (2013) Detection limits of DLS and UV-vis spectroscopy in characterization of polydisperse nanoparticles colloids. Journal of Nanomaterials 60: 60.
© 2022 Jeff Whitworth. This is an open access article distributed under the terms of the Creative Commons Attribution License , which permits unrestricted use, distribution, and build upon your work non-commercially.
 a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in
a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in 







.jpg)






























 Editorial Board Registrations
Editorial Board Registrations Submit your Article
Submit your Article Refer a Friend
Refer a Friend Advertise With Us
Advertise With Us
.jpg)






.jpg)














.bmp)
.jpg)
.png)
.jpg)










.jpg)






.png)

.png)



.png)






