- Submissions

Full Text
Modern Concepts & Developments in Agronomy
Investigation of R-Leaf Technology as a New Source of Nitrogen Fertiliser for Crop Yield and Productivity-A Field Trial
Rahul Ramalingam2, Ramana Sundara2, Sankeerthana Bellamkonda1* and Apostolos Papadopoulos1*
1Crop Intellect Limited, United Kingdom
2Lincoln Institute for Agri-Food Technology (LIAT), United Kingdom
*Corresponding author: Sankeerthana Bellamkonda and Apostolos Papadopoulos Crop Intellect Limited, University of Lincoln, United Kingdom
Submission: August 16, 2022;Published: October 11, 2022

ISSN 2637-7659Volume11 Issue 4
Abstract
Nitrogen is an essential nutrient for plants and a significant component of proteins, which all animals need to grow, reproduce and survive. Nitrogen is often the nutrient that is limiting to increase crop production, despite Earth’s atmosphere containing more than 78 percent. This is because in the atmosphere, nitrogen largely exists in its unreactive N2 form, rather than in a reactive form which plants can utilize. There are a number of scientific and technological innovations which have allowed for rapid growth in crop productivity, particularly in the second half of the 20th century. None of these had a more dramatic impact than the ability to produce synthetic nitrogen fertiliser. In this context, Crop Intellect Ltd has invented a disruptive technology called R-Leaf that captures nitrogen oxides (NOx) pollution from the atmosphere and converts it to nitrates to feed plants. In principle, R-Leaf photocatalysts break down the nitrogen oxides to nitrate on the surface of the plants which is then absorbed as fertiliser. The authors have carried out field trials investigating R-Leaf sprayed (at 1L/ha) over an area of lawn at the University of Lincoln, UK. In accordance with the results of the completed investigations, it has been confirmed that the application of R-Leaf on the grass has helped to improve the crop yield by 13 -20%, which is corroborated with sap nitrate, leaf chlorophyll and growth measurements.
Keywords: Nitrogen; Fertilisers; Air pollution; R-Leaf; Photocatalysis; Crop yield
Introduction
Fertilisers have become essential to improve the crops’ yield and nutritional quality especially after the progression of fertiliser responsive crop varieties. Nitrogen is the most important component for supporting plant growth. Nitrogen is part of the chlorophyll molecule, which gives plants their green color and is involved in creating food for the plant through photosynthesis. Without enough nitrogen in the plant, the plant cannot grow tall, or produce enough food. Thus, it plays a significant role during the vegetative growth of crops. Nitrogen is absorbed by the plants in the form of nitrate (NO3−) and ammonium (NH4+) species. However, the same nitrogen can be lost through the processes of nitrate leaching, denitrification, and ammonia volatilization. Low nitrogen availability in soil primarily limits the growth and yield of plants. Therefore, the practice of applying inorganic nitrogen fertiliser has been adopted globally to increase crop yield and farming profitability. At the same time, the manufacture and use of nitrogen fertiliser have come up with a wide range of environmental issues such as a drop in biodiversity in and out of agricultural systems, eutrophication of freshwaters, estuaries, and coastal water, emission of greenhouse gases into the atmosphere and land habitats deficient in nutrition. Nitrates specifically can be leached easily from agricultural lands [1]. Loss of these mineral nutrients through leaching and runoff to surface and ground water along with abundant volatilization constitute growing concerns due to economic losses and environmental pollution. Nitrogen volatilization results in the release of nitrogen oxides (NOx including N2O) and thus contributing to global warming as greenhouse gases.
The contribution of NO emissions from agricultural soils has previously not been a major focus due to the dominance of vehicles and power generation and other industry as NO sources in the UK. However, as the combustion sources are projected to decrease, the agricultural share of the UK’s total NOx emissions through the soil, NO emissions are expected to increase (currently estimated at 4% and projected to increase to 6% by 2030) [2]. A recent study suggests that soil NO emissions may be larger than previously thought, being estimated to account for 20-32% of NOx emissions in California [3]. Soil nitrogen compounds are also reduced to nitrous oxide ( N2O), a powerful greenhouse gas [4]. In this regard, the use of nitrogen fertilisers and livestock waste in agriculture is the main contributor to UK N2O emissions, accounting for 80% of N2O emissions in the UK [5]. Thus, a wider role of agriculture in air pollution generally, and in greenhouse gas emissions and climate change, also needs to be considered [6]. To achieve significant emission reductions of the UK’s N2O, mitigation action in the agricultural sector would be required.
In industry, efforts have traditionally focused on reducing the total amount of N2O emitted. For instance, technologies have been developed that lead to cleaner combustion and/or reduce N2O from the waste gases of combustion chambers. Cleaner energy sources are also being heavily invested in for the same reasons. However, these technologies and energy sources are currently not effective enough to reduce the N2O output of industry to acceptable levels.
In agriculture, efforts have focused on reducing fertiliser application, developing less harmful fertilisers, growing perennial crops instead of annual crops, developing expertise regarding the timing of fertiliser application, and introducing buffer zones near known pollution areas [7-9]. These measures have all been shown to have some impact on reducing the effect of nitrogen pollution in the natural environment. However, they are expensive to implement and often have drawbacks associated with them (such as reduced crop yield and/or crop quality). As the world’s population is estimated to increase to approximately 9.8 billion people by the year 2050, such solutions are often not economically feasible.
Crop Intellect has invented a technology called R-Leaf that oxidizes NOx into N-fertiliser (nitrates) by absorbing daylight energy through photocatalysis. The formulation is tank mixed and sprayed over foliage during normal farming schedules. R-Leaf consists of a specially processed titanium dioxide (TiO2) to work under normal light, in a cost-effective manner to be used in agriculture. It enables farmers to produce sustainable N- fertiliser from atmospheric NOx as substrates under daylight resulting in crop growth and yield enhancement.
How R-Leaf® Technology Works
R-Leaf® technology captures atmospheric NOx pollutants and convert them into plant feed. NOx is broken down into NO3- and is taken up by plants as feed resulting in lowered air pollution and improved crop yield. R-Leaf® incorporates photocatalysts that are particularly designed to absorb NOx under normal sunlight i.e., visible light instead of UV light. The R-Leaf® particles stay on the surface of the plants on which they are sprayed and work continuously in breaking down NOx into nitrate which is the most desired form of nitrogen for plants. The nitrate formed gets dissolved in dew and rain and absorbed by the plants that subsequently leads to an increase in biomass production and yield.
Working Principle: When R-Leaf® is exposed to sunlight, electrons and holes are generated. The electrons (e-) which are negatively charged attract oxygen from the atmosphere and form superoxide anions (O2sub>.-). The positively charged holes (h+) interact with water molecules in the atmosphere and forms hydroxyl radicals (. OH). When the NOx gases in the atmosphere react with the formed superoxide and hydroxyl radicals, NOx is broken into nitrate (NO3-) and water (H2O), necessary for plant growth.
Figure 1:(A) Section 1 and (B) Section 2 of lawn/grass at Riseholme College, LN2 2LG, UK.
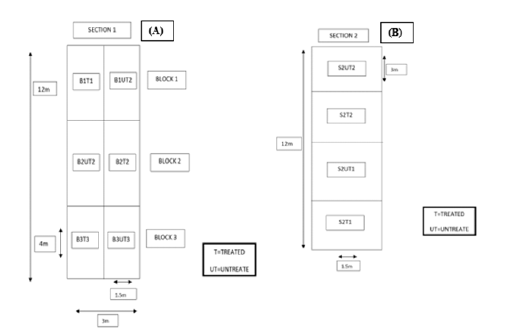
An area of standard lawn (grass) at the University of Lincoln, Riseholme college, UK was chosen for a trial and separated into two sections, Section 1 and Section 2. In order to verify the crop productivity using R-Leaf fertiliser, Section 1 was used to measure crop growth and Section 2 was used to take chlorophyll and nitrate measurements (destructive). Furthermore, both the sections are segregated as treated and untreated blocks/sub-sections using Randomized Block Design as shown in Figure 1.
Randomized block design
The Randomized Block Design (RBD) is one of the most widely applied field trial formats. Employment of blocks in the fields using RBD improves the accuracy of the investigation as the variations in the plots can be minimized by clustering them collectively. For instance, the plots with slope and soil depth differences can be clustered together accordingly. Moreover, it is reasonably facile to evaluate as far as missing value points are avoided [10]. Prior to the application of R-Leaf fertiliser, both sections of the grass were trimmed by using a standard Lawn Mower and ensured that the grass height is consistent. The total area of Section 1 (12×3) is divided into three blocks as six plots each of 4×1.5m area as shown in Figure 1A and the total area of Section 2 (12×1.5m) is divide into four plots each of 3×1.5m area. Each plot was marked using canes in both sections. The recommended dosage of 1 L of R-Leaf (per hectare) was applied by using the equivalent of 200 L of water (manufacturer’s recommendation). Accordingly, the total amount of R-Leaf required for spraying was calculated as shown below.
Section 1: The total amount of R-Leaf required for spraying is calculated as follows.
Calculation:
1 L R-Leaf with 200 L of water for 1 hectare.
Each plot consists of = length-4m, breadth - 1.5m.
Covert to square meter = 4m × 1.5m = 6m2.
The required volume of R-Leaf needed for 6m2 is calculated as
follows:
X * 1L = 1 hec * 6m2
X * 1000mL = 10000m2
X = 1000mL % 10000m2
X = 0.1 * 6m2
X = 0.6mL
0.6mL is used for 6m2.
The required volume of water needed for 0.6 mL is:
X * 200L water = 1L of R-Leaf × 0.6mL
X * 200000mL = 1000mL × 0.6mL
X = (200000/1000) × 0.6mL
X = 200 × 0.6mL
X = 120mL of water.
Hence the required dosage is 0.6mL of R-Leaf and 120mL of water for 6m2 area.
Section 2: The total amount of R-Leaf required in Section 2 for spraying is as follows.
Calculation:
1 L R-Leaf with 200 L of water for 1 hectare.
Each plot consists of = length -3m, breadth -1.5m.
Area = 3m × 1.5m = 4.5m2.
Volume of R-Leaf needed for 4.5m2 is
X × 1L = 1hec × 4.5m2
X × 1000mL = 10000m2
X = 100mL/10000m2
X = 0.1 × 4.5m2
X = 0.45mL
0.45mL of R-Leaf is used for 4.5m2.
Volume of water needed for 0.45mL R-Leaf fertiliser is:
X × 200L water = 1L of R-Leaf × 0.45mL
X × 200000mL = 1000mL × 0.45mL
X = (200000/1000) × 0.45mL
X = 200 × 0.45mL
X = 90mL of water.
Hence the needed dosage is 0.45mL of R-Leaf and 90mL of water for 4.5m2.
Correspondingly, the total amount of R-Leaf needed in both Section 1 and Section 2 is 1.8mL in 360mL water. Totally, 3.6mL of R-Leaf in 720mL water is required to spray both the sections. The Berthoud (16L) sprayer with MV 80° and RS 80° nozzle has been used for spraying. The operator familiarised with the volume sprayed at pace to achieve the required dosage. The R-Leaf has been applied on 16th July 2021 once, and taken measurements of chlorophyll, nitrate and growth measurements on 20th July 23rd July 27th July and 3rd of August 2021.
Measurements
Chlorophyll measurement
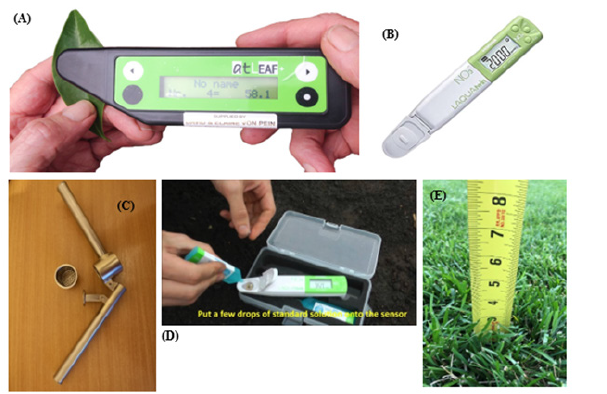
Chlorophyll measurements in Section 2 were performed using at LEAF chlorophyll meter (Figure 2A). In total, 10 samples were collected from each plot and placed in separate bags by noting the plot number: and measured chlorophyll by inserting each sample as shown in (Figure 2A). Readings were recorded and noted manually and where measurements were higher than 75 or lower than 20 were discarded.
Nitrate measurement
Nitrate measurements in Section 2 were performed using a calibrated HORIBA LAQUATWIN meter (Figure 2B). 30 samples were collected from each plot by using a garlic press to squeeze out sap (Figure 2C) and place on the sensor (Figure 2D). The results of nitrate content were given in ppm.
Growth measurement
Height measurements from Section 1 were recorded using a regular ruler as shown in (Figure 2E). Ten measurements were taken from each plot at each time point for both treated and untreated.
Figure 2:(A) Chlorophyll meter; (B) Nitrate meter; (C) Garlic Press/Sap Squeezer; (D) Nitrate meter calibration (E) Growth measurement using ruler.
Results and Discussion
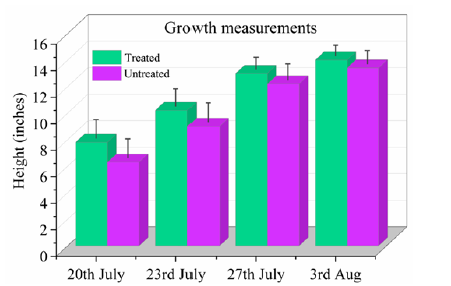
The height measurements of grass crop between treated and untreated on selective dates (20th July (4 days after application), 23rd July 27th July and 3rd Aug of 2021) were measured and shown Figure 3. It shows that the grass treated with R-Leaf has grown nearly 13% more than that of the untreated in Section 1 over one week after application. This could be due to a higher intake of nitrate by plants which in turn increased chlorophyll content in plants. This is further reverified by measuring nitrate and chlorophyll contents in the treated and untreated grass later in the report.
Figure 3:Growth measurements on R-Leaf treated and untreated blocks in Section 1 grass crop.
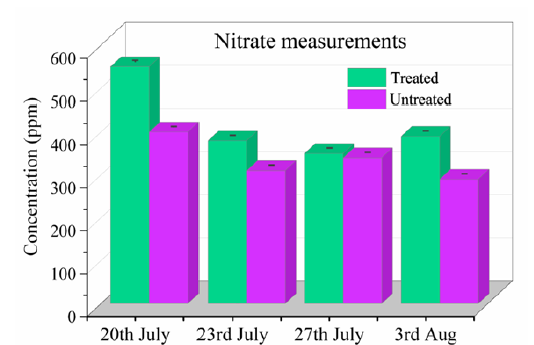
The nitrate measurements between R-Leaf fertiliser treated and untreated grass are shown in Figure 4. The results showed that R-Leaf treated (551.67) grass was greater compared to the untreated (410) on 20th July 2021 in terms of nitrate content (ppm). The results further revealed that the treated grass had consistently higher nitrate content in the R-Leaf fertiliser treated grass compared to the control.
Figure 4 reveals that nitrate content in treated area is much greater than untreated on the 4th day after application (20 July 2021) and also significantly different in the last measurement (3 August 2021) compared to the two middle measurements (23rd and 27th July). It is confirmed that R-Leaf comprising titanium dioxide photocatalyst particles specially processed by Crop Intellect’s invention, produced nitrate which solubilized in dew present in the morning on the foliage and it was absorbed by the grass plants resulting in increased biomass derived from growth characterisation. The photocatalysts therefore are ready to produce more nitrate the following day having been cleared of produced nitrate. Therefore, the application of R-Leaf fertiliser on the plants provides a cost-effective way of improving crop yield/crop growth as it is required only a few times per year. This method provides the crop with nitrates for a period of around 45 days per application during growth.
Figure 4:Nitrate measurements on R-Leaf treated and untreated blocks in Section 2 grass crop.
Nitrogen promotes rapid plant growth and raises the protein concentration of fodder crops; it also promotes the uptake and utilisation of all other nutrients such as potassium and phosphorus, and regulates total crop growth [11,12]. Reduced nitrogen efficiency results in reduced development, chlorosis (leaf colour changes from green to yellow), as well as the emergence of red and purple patches on foliage. Typically, deficiency symptoms occur on the mature leaves. Excessive usage of nitrogen promotes excessive vegetative growth, especially in tropical climates. Plants can absorb nitrogen that is advantageous to them; many plants absorb nitrogen in the nitrate form, but this is ineffective in some soils, such as flooded soils, while NH4+ is the most suited and permanent form for paddy soils. Optimal amount of nitrogen is required for optimal plant growth and development which vary depending on plant type. Insufficient nitrogen treatment affects crop output, whereas too much nitrogen has detrimental effect on plant growth, and this issue is becoming increasingly important in crop yields [13].
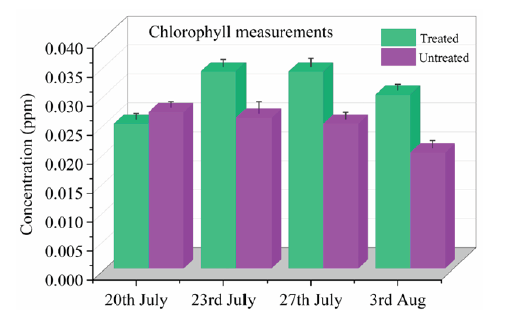
The chlorophyll measurements of the Section 2 of grass treated and untreated are shown in Figure 5. According to experimental results, the chlorophyll content in treated subsection of the grass is slightly lower (0.024825) compared to untreated area (0.0262045) on the 20th of July. However, on the next three days the chlorophyll content is higher in treated area than the untreated. As we know, plants in general absorb nitrates as a source of nitrogen which is further required to produce proteins necessary for growth. The quantification of chlorophyll content in plants is directly related to the amounts of nitrates. Accordingly, when the R-Leaf formulation is applied on the grass, both the amounts of nitrates and chlorophyll increased which can be seen in the form of crop yield, as shown in Figure 3.
Wen et al. [14] and Guo et al. [15] have already proved that nitrates are the source of chlorophyll improvement in the plants. The quantification of chlorophyll is a critical measure for confirming the number of pigments engaged in light absorption and energy conversion during photosynthesis. The decrease in chlorophyll concentration might be an identification of plant ageing. Nitrogen is the most vital component present in chlorophyll molecules, increased the photosynthetic efficiency. From Figure 5, overall chlorophyll content in grass has increased significantly from the second to the last day of data collection. Overall, according to our experimental results and field trials, the R-Leaf formulation containing the specially processed TiO2 photocatalyst is a novel and suitable way to enhance the nitrogen and chlorophyll contents in grass thereby improving the crop productivity and biomass.
Figure 5:Chlorophyll measurements on R-Leaf treated and untreated blocks in Section 2 grass crop.
Conclusion
Crop Intellect’s R-Leaf technology has been developed with the aim of converting NOx pollutants into a natural N-fertiliser by absorbing solar energy. The results obtained confirm that the application of R-Leaf on the grass improved crop yield, which is corroborated with nitrate, chlorophyll and growth measurements taken over two weeks after application. It can therefore be concluded that R-Leaf can be used in grass to support growth through the supply of nitrate to the plants for at least two weeks after application whilst removing NOx air pollution.
References
- Andrews M, Raven JA, Lea PJ (2013) Do plants need nitrate? The mechanisms by which nitrogen form affects plants. Annals of Applied Biology 163(2): 174-199.
- Sutton MA, Howard CM, Brownlie WJ, Skiba U, Hicks WK, et al. (2017) The European Nitrogen Assessment 6 years after: What was the outcome and what are the future research challenges? In: Innovative Solutions for Sustainable Management of Nitrogen. Procs. From the International Conference, Aarhus, Denmark.
- Almaraz M, Bai E, Wang C, Trousdell J, Conley S, et al. (2018) Agriculture is a major source of NOx pollution in California. Science Advances 4(1): 1-8.
- Harter J, Guzman-Bustamante I, Kuehfuss S, Ruser R, Well R, et al. (2016) Gas entrapment and microbial N2O reduction reduce N2O emissions from a biochar-amended sandy clay loam soil. Scientific Reports 6: 39574.
- EEA (2010) Annual European Union greenhouse gas inventory 1990–2008 and inventory report 2010. EEA Technical Report no. 6/2010. Copenhagen, Denmark.
- Skiba U, Ball B (2002) The effect of soil texture and soil drainage on emissions of nitric oxide and nitrous oxide. Soil Use and Management 18(1): 56-60.
- Skowroñska M, Filipek T (2014) Life cycle assessment of fertilizers: a review. International Agrophysics 28:101-110.
- Yan W, Zhisheng Y, Xunhua Z, Logapragasan S, Klaus BB (2022) A synthesis of nitric oxide emissions across global fertilized croplands from crop-specific emission factors. Global Change Biology 28(14): 4395-4408.
- Eric AD, Mark BD, James NG, Christine LG, Richard H, et al. (2012) Excess Nitrogen in the U.S. Environment: Trends, Risks, and Solutions. Issues in Ecology by The Ecological Society of America 15: 1-16.
- Norman DW (1995) The farming systems approach to development and appropriate technology generation. Food & Agriculture Org. (No. 10).
- Sheehy JE, Gastal F, DURAND JL, Lemaire G, Woodward FI (1996) A nitrogen-led model of grass growth. Annals of Botany 77: 165-177.
- Leghari SJ, Wahocho NA, Laghari GM, Hafeez LA, Mustafa BG, et al. (2016) Role of nitrogen for plant growth and development: A review. Advances in Environmental Biology 10(9): 209-219.
- Shi D, Zhuang K, Chen Y, Xu F, Hu Z, et al. (2020) Effects of excess ammoniacal nitrogen (NH4+- N) on pigments, photosynthetic rates, chloroplast ultrastructure, proteomics, formation of reactive oxygen species and enzymatic activity in submerged plant Hydrilla verticillata (Lf) Royle. Aquatic Toxicology 226: 105585.
- Wen B, Li C, Fu X, Li D, Li L, et al. (2019) Effects of nitrate deficiency on nitrate assimilation and chlorophyll synthesis of detached apple leaves. Plant Physiology and Biochemistry 142: 363 -371.
- Guo S, Zhou Y, Shen Q, Zhang F (2007) Effect of ammonium and nitrate nutrition on some physiological processes in higher plants-growth, photosynthesis, photorespiration, and water relations. Plant Biology 9(1): 21-29.
© 2022 Apostolos Papadopoulos. This is an open access article distributed under the terms of the Creative Commons Attribution License , which permits unrestricted use, distribution, and build upon your work non-commercially.
 a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in
a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in 







.jpg)






























 Editorial Board Registrations
Editorial Board Registrations Submit your Article
Submit your Article Refer a Friend
Refer a Friend Advertise With Us
Advertise With Us
.jpg)






.jpg)














.bmp)
.jpg)
.png)
.jpg)










.jpg)






.png)

.png)



.png)






