- Submissions

Full Text
Modern Concepts & Developments in Agronomy
Enhancement of Seed Potential under Forced Ageing using Selected Plant Extract
Chandan Kumar Pati*
Department of Botany, Saldiha College (Affiliated to Bankura Univesity), Bankura, West Bengal, India
*Corresponding author: Chandan Kumar Pati, Department of Botany, Saldiha College (Affiliated to Bankura Univesity), Bankura, West Bengal, India
Submission: August 15, 2022;Published: September 08, 2022

ISSN 2637-7659Volume11 Issue 3
Abstract
Horse-gram seeds was pretreated with aqueous solution of leaf extract of bel (Aegle marmelos) 50g in 500ml distilled water for 2 hours before forced ageing treatment (100% RH and 32±2 °C) for different durations (0 and 30 days). Seeds showed better performance, measured in terms of few physiological and biochemical parameters after pretreatment. The effect of the herbal extract on enhancement of seed potential is apparent in this investigation.
Keywords:Bel; Horse-gram; Seed potential; Forced ageing
Abbreviations: PSG: Percentage Seed Germination; PR: Protein; CAT: Catalase; RH: Relative Humidity; ISTA: International Seed Testing Association
Introduction
Horse-gram (Dolichos biflorus L.) is one of the highly nutritious vegetable pulse crops and has some ethno medicinal values. Traditionally the aqueous extracts of seed are used to cure the urinary troubles, sunburn, kidney stone, female diseases (leucorrhoea, menstrual troubles, bleeding during pregnancy, post-partum excessive discharges), hemorrhagic disease, intestinal worms etc. [1,2]. But the semiarid climate and High Relative Humidity (RH) prevailing during a major part of a year strongly impair seed storage potential in Indian agroclimatic condition [3-6]. In view of the said problem, the main objective of the present work was to probe the efficacy of the bel plant extract on enhancement of seed potential of a horse-gram species
Materials and Methods
Immediate after surface sterilization with 0.1% HgCl2 for 90 seconds, the seed sample of horse-gram (Dolichos biflorus L. cv BR-5) was presoaked with aqueous solution of leaf extract of bel (Aegle marmelos) 50g in 500 ml distilled water for 2 hours and then dried back to the original dry weight of the seeds. The pretreated seed lot was taken in separate cloth bag and stored in a desiccator in which 100% Relative Humidity (RH) was provided. This experimental set up was kept at 32±2 °C for 30 days allowing the seeds to experience forced ageing treatment.
To study the seed potential few parameters viz. percentage seed germination [7], protein [8] level as well as activity of catalase [9] enzyme of seed kernels was analysed from the zeroand 30-day ageing seeds. Data were statistically analysed following the methods of [10].
Results and Discussion
Seeds pretreated with aqueous solution of leaf extract of bel (Aegle marmelos) slowed down the rapid loss of germination and significantly alleviated the forced ageing-induced loss of protein level as well as activity of catalase enzyme of seed kernels (Table 1).
Table 1: Effect of seed pretreatment with aqueous solution of leaf extract of bel (Aegle marmelos) 50g in 500ml distilled water on Percentage Seed Germination (PSG), level of protein (PR; mg/g fr. wt.) and catalase activity (CAT; ΔOD x Tv/txv) from horse-gram seeds which underwent forced ageing for 0 and 30 days.
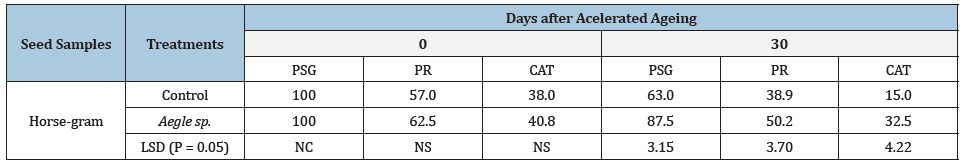
NC: Not calculated; NS: Not significant.
Thus, the results indicated that although deterioration is a common phenomenon in treated and control samples of the seed species, the catabolic processes within the treated seed sample remained somewhat subdued, thereby rendering them tolerant against unfavorable storage environment [11,12]. Catalase is regarded as a scavenger enzyme [13] and its higher activity is indicative of higher seed vigour [14-18].
Conclusion
It can be concluded from the results of this investigation that the promising effect of the aqueous solution of leaf extract of Aegle marmelos on enhancement of seed potential of horse-gram species is much significant. Thus, invigouration property of the present seed pretreating agent seems to be apparent from these experimental results.
References
- Laskar S, Bhattacharyya UK, Sinhababu A, Basak BK (1988) Antihepatoxic activity of Kulthi seeds in rats. Fitoterapea 69(5): 401-402.
- Mishra VK (2006) Studies on seed vigour and viability of a few pulse crops and devising techniques of seed invigouration. Ph.D. Thesis, Vidyasagar University, India.
- Christensen CM, Kaufmann HH (1965) Deterioration of stored grain by fungi. Annal Review of Phytopathology 3: 69-84.
- Aziz NH, Shair AAM (1997) Influence of other fungi on aflatoxin production by Aspergillus flavus in maize kernels. Journal of Food Safety 17(2): 113-123.
- Copeland LO, McDonald MB (1995) In principles of seed science and technology. (3rd edn), Chapman and Hall, New York, USA.
- Pati CK (2019) Seed potentition and enhancement of plant potential of a mungbean species using plant extracts. World Scientific News 131:268-271.
- International Seed Testing Association (1976) International Rules for Seed Testing. Seed Science and Technology 4: 51-177.
- Lowry OH, Rosebrough NJ, Farr AL, Randall RJ (1951) Protein measurement with the Folin-phenol reagent. Journal of Biological Chemistry 193(1): 265-275.
- Snell FD, Snell CT (1971) Colorimetric methods of analysis, Van Nostrand Reinhold Co., New York, USA, 4AAA: 7-145.
- Panse VG, Sukhatme PT (1967) Statistical methods for agricultural workers. Indian Council of Agricultural Research, New Delhi, India, 2: 150-157.
- Abdul-Baki AA, Anderson JD (1972) Physiological and biochemical deterioration of seeds. In: Kozlowski TT (Ed.), Seed Biology, Academic Press, New York, USA, 2: 283-315.
- Kole S, Gupta, K (1982) Biochemical changes in safflower (Carthamus tinctorius) seeds under accelerated ageing. Seed Science and Technology 10(1): 47-54.
- Fridovich I (1976) Oxygen radicals, hydrogen peroxide, and oxygen toxicity. In Prior WA (Ed.), Free Radicals in Biology, Academic Press, New York, 1: 239-277.
- Basu RN (1994) An appraisal of research on wet and dry physiological seed treatments and their applicability with special reference to tropical and sub-tropical countries. Seed Science and Technology 22(1): 107-126.
- Pati CK, Bhattacharjee A (2011) Alleviation of seed deterioration and enhancement of plant potential of a pea species using selected medicinal plant extracts. Journal of Medicinal Plant Research 5(17): 4108-4111.
- Ojha S, Pati CK, Sen M (2020) Allelopathic effect of Fagopyrum plant extracts on crop seed species. Science and Culture 86(3-4):113-115.
- Arnon DI (1949) Copper enzymes in isolated chloroplasts. Polyphenol oxidase in Beta vulgaris. Plant Physiology 24(1): 1-15.
- McCready RM, Guggloz J, Silvirea V, Ownes HS (1950) Determination of starch and amylase in vegetables. Analytical Chemistry 22(9): 156-1158.
© 2022 Chandan Kumar Pati. This is an open access article distributed under the terms of the Creative Commons Attribution License , which permits unrestricted use, distribution, and build upon your work non-commercially.
 a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in
a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in 







.jpg)






























 Editorial Board Registrations
Editorial Board Registrations Submit your Article
Submit your Article Refer a Friend
Refer a Friend Advertise With Us
Advertise With Us
.jpg)






.jpg)














.bmp)
.jpg)
.png)
.jpg)










.jpg)






.png)

.png)



.png)






