- Submissions

Full Text
Modern Concepts & Developments in Agronomy
Synergistic Complementary Effects of Elemental Sulfur and Micro-Algae on Saline- Alkali Soils
Liming Lai1* and Jiuhuan Feng2
1Department of Agronomy, Hetao College, China
2Sanrui Agritec Co. Ltd., China
*Corresponding author:Liming Lai, Department of Agronomy, Hetao College, Bayannur, Inner Mongolia 015000, China
Submission: August 11, 2022;Published: August 25, 2022

ISSN 2637-7659Volume11 Issue 3
Abstract
Sunflower (Helianthus annuus L.) is one of the major crops in the Hetao Irrigation District (HID), which belongs to the cold and arid region, Bayannur, Inner Mongolia, China. The sunflower is planted in the saline-alkali farmlands, accounting for approximately 1/2 of the total planting area. The sunflower yield is relatively low. However, studies on improving the saline-alkali soils in the HID are still lacking. In this study, through an in-depth investigation of the HID soils and literature review, we revealed the synergistic complementary effects between elemental sulfur (S0) and Micro-Algae (MA) on saline-alkali soils. Also, we proposed a novel idea that the interaction between S0 and MA could be used to improve the salinealkali soils in the HID or other arid regions.
Keywords: Sunflower; Elemental sulfur; Micro-algae; Saline-alkali soil; Hetao irrigation District
Introduction
The Hetao Irrigation District (HID) (40°12’-41°20’ N, 106°10’-109°30’ E) is located in Bayannur, Inner Mongolia, China. The HID belongs to the cold and arid region, with 0.68 million ha of irrigated arable lands. The HID farmland soil was classified as Irrigation Silting Soil by China Soil System Classification, which is similar to Plaggept by the US Soil Taxonomy. The soils are alkaline with low Soil Organic Matter (SOM) content. Also, the soils have interannual periodic secondary salinization [1]. Owing to its salt tolerance, the sunflower (Helianthus annuus L.) is planted in the saline-alkali lands of the HID, accounting for approximately 1/2 of the total arable lands in the district. The sunflower yield is relatively low, the annual mean yield from 2000 to 2018 was only 2370 kg ha-1[2]. However, research studies on improving the saline-alkali soils are still lacking. Through literature review and field investigation, we revealed the synergistic complementary effects of elemental Sulfur (S0) and Micro-Algae (MA) (as bio-fertilizers) on the saline-alkali soils. We also proposed that the combination of S0 and MA could be used to improve the saline-alkali soils. This study would shed new light on improving saline-alkali soils in the HID or other arid regions of the world.
Discussion
S0 application
Can effectively reduce the pH value of saline-alkali soils. Firstly, S0 can be oxidized by microorganisms to generate sulfate ions (SO42-) and hydrogen ions (H+), which can reduce the soil pH value. Also, S0 is insoluble in water, resulting in a slow microbial oxidation process, which keeps the lower pH value for a longer time [3-5]. The decrease in pH value of the alkaline soil can dissolve the insoluble calcium carbonate (CaCO2) in the soil. The dissolved CaCO2 can replace the soil exchangeable Na+, thereby reducing the ESP in the soil [6].
MA commonly refers to the collective name of single-celled micro-algae that contain chlorophyll-a, which are capable of photosynthesis. MA belongs to a kind of protists. A variety of soil bio-fertilizers have been developed by using different MA species as raw materials. The bio-fertilizers were commonly used in paddy fields previously, but currently, in dryland, the bio-fertilizer application is increasing [16]. a) MA as bio-fertilizers can reduce soil sulfur enrichment. Cyanobacteria (one of the algae species) first appeared in the Archean oceans on the Earth. With increasing oxygenation and more abundant sulfate (SO42-) levels, algae (green algae together with cyanobacteria) became the main primary producers in the Earth’s oceans. Sulfur is one of the important components of algae cells. The sulfur is consumed by algae in the form of SO4 2-. Therefore, sulfur has become a macronutrient necessary for the growth of micro-algae [17]. The application of MA bio-fertilizers can inevitably consume part of SO4 2- and reduce sulfur enrichment and sulfate content in the soil. b) The cyanobacteria integrated with salt-tolerant plants can remove much more salts from soils [18]. The major contribution of cyanobacteria is to re-establish micro-ecology [18] and absorb Na+ in the soil [19]. c) MA bio-fertilizers can increase soil availability of nutrients [16,20]. In drylands, MA species can grow in symbiosis with crop roots [21], significantly affecting microbial communities [19,22], improve plant rhizosphere microbes [23-25], decompose soil compounds to generate available nutrients [21], enhance crop N uptake, and fix N [16]. d) MA can increase Soil Organic Carbon (SOC) through photosynthesis [16], thereby increasing SOM. For example, the application of MA biofertilizers in desert soil in the United States has been reported to increase SOM from 10 to 30 g kg-1 within three years [26]. e) MA bio-fertilizers can significantly reduce N2O emissions from the soils [27].
However, little information is available on the interaction between S0 and MA. To our knowledge, only one study has so far evaluated the impacts of sulfur and algae fertilization on the productivity of soybean (Glycine max (L.) Merrill) and mung bean (Vigna radiata (L.) Wilczek) in Egypt. The results showed that compared with the single application of S0, MA, or the control, under the condition of combined application of S0 and MA, the yields of soybean and mung bean were significantly increased, and the protein, carbohydrate, and oil contents also increased accordingly [12]. However, the effects of S0-MA interaction on soils have not been reported yet.
Conclusions
As a fertilizer, S0 can reduce soil pH and ESP, improve N nutrient availability in the soil, and reduce soil NO3--N leaching, but can lead to soil sulfur enrichment (i.e., increase in soil SO42- content) and an increase in soil total salt content (Table 1). Whereas MA as bio-fertilizers can significantly reduce soil SO42- content, soil total salt content, soil N2O emission, can improve SOM content and fix N in the soil (Table 1). Therefore, the S0 and MA have strong synergistic and complementary effects on saline-alkali soil, by which we proposed a novel idea that the interaction between S0 and MA could be used to improve the saline-alkali soils in the HID or other globally arid regions.
Table 1:Synergistic complementary effects of elemental sulfur and micro-algae as bio-fertilizers on saline-alkali soils.
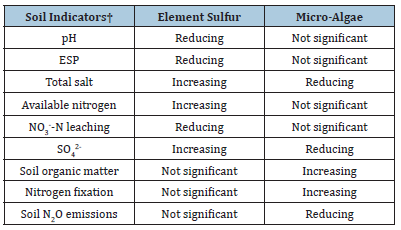
† ESP, Exchange sodium percentage; NO3--N, Nitrate nitrogen; SO42-, Sulfate ion; N2O, Nitrous oxide.
Acknowledgment
This study was financially supported by the Inner Mongolia Natural Science Foundation (NO. 2020MS04001), the Research Funding Project of Hetao College Talent Introduction (NO. HYRC2019006), and the Hetao College Science and Technology Research Project (NO. HYZX201952).
References
- Lai L, Mei L, Yang Y (2022) Agricultural soil characteristics and development in hetao irrigation district, Inner Mongolia. Jiangsu Agriculture Science 50(2): 213-218.
- Inner Mongolia Autonomous Regional Bureau of Statistics (2019) Inner Mongolia Statistics Yearbook 2019, China Statistics Press, Beijing, China.
- Nor YM Tabatabai M (1977) Oxidation of elemental sulfur in soils. Soil Science Society of America Journal 41(4): 736-741.
- Turan MA, Taban S, Katkat AV, Kucukyumuk Z (2013) The evaluation of the elemental sulfur and gypsum effect on soil pH, EC, SO4-S and available Mn content. Journal of Food, Agriculture & Environment 11(1): 572-575.
- Slaton NA, Norman RJ, Gilmour JT (2001) Oxidation rates of commercial elemental sulfur products applied to an alkaline silt loam from Arkansas. Soil Science Society of America Journal 65(1): 239-243.
- Liu G, Li X, Zhang Y, Lu J, Wei Y (2008) Primary study on function of sulfur on saline-alkali soil in Yinbei Area. Agricultural Research in the Arid Areas 26(04): 79-82.
- McCauley A, Jones C, Olson-Rutz K (2017) Nutrient Management Module No. 8: Soil pH and organic matter-4449-8. MSU Extension, Montana Stete University (MSU), USA.
- Neina D (2019) The role of soil pH in plant nutrition and soil remediation. Applied and Environmental Soil Science, Article ID: 5794869.
- Zareabyaneh H Bayatvarkeshi M (2015) Effects of slow-release fertilizers on nitrate leaching, its distribution in soil profile, N-use efficiency, and yield in potato crop. Environmental Earth Sciences 74(4): 3385-3393.
- Liu C, Zhao Y, Hu Z, Gao Y, Xu C, Bi D, et al. (2004) Effect of element sulphur on the leaching loss of NO3- from vegetable soil. Journal of Nanjing Agricultural University 27(3): 54-57.
- Sun Z, Wu Z, Chen L, Jia L (2008) Regulation of soil nitrification with nitrification inhibitors and related mechanisms. Chinese Journal of Applied Ecology 19(6): 1389-1395.
- Essa R, Afifi A, Ashry S (2021) Influence of sulfur coated urea and algae fertilization on productivity of some leguminous crops in sandy soils. Bulletin of the National Research Centre 45(60): 61-69.
- Hasanuzzaman M, Hossain MS, Bhuyan MB, Al Mahmud J, Nahar K, et al. (2018) The role of sulfur in plant abiotic stress tolerance: molecular interactions and defense mechanisms. Plant Nutrients and Abiotic Stress Tolerance, Springer Nature Singapore Pte Ltd., Singapore, pp. 221-252.
- Lu Y, Wang Qf, Li J, Xiong J, Zhou Ln, et al. (2019) Effects of exogenous sulfur on alleviating cadmium stress in tartary buckwheat. Scientific Reports 9(1): 1-12.
- Demir I Basalma D (2018) Response of different level of nitrogen and sulphur doses on oil yield and seed nutrients content of sunflower (Helianthus annuus L.). Fresenius Environmental Bulletin 27(9): 6337-6342.
- Alvarez AL, Weyers SL, Goemann HM, Peyton BM, Gardner RD (2021) Microalgae, soil and plants: A critical review of microalgae as renewable resources for agriculture. Algal Research 54: 102200.
- Giordano M Prioretti L (2016) Sulphur and algae: metabolism, ecology and evolution. In: Borowitzka M, Beardall J, et al. (Eds.), The Physiology of Microalgae, Developments in Applied Phycology 6, Springer International Publishing, Switzerland.
- Jesus JM, Danko AS, Fiúza A, Borges M-T (2015) Phytoremediation of salt-affected soils: a review of processes, applicability, and the impact of climate change. Environmental Science and Pollution Research 22(9): 6511-6525.
- Li H, Zhao Q, Huang H (2019) Current states and challenges of salt-affected soil remediation by cyanobacteria. Science of the Total Environment 669: 258-272.
- Jacob-Lopes E, Maroneze MM, Queiroz MI, Zepka LQ (2020) Handbook of microalgae-based processes and products, Academic Press, Elsevier Inc., London, UK; San Diego, CA, US; Cambridge, MA, US; Oxford, UK
- Algae Planet (2021) Algae Basics. Algae Innovations Media, LLC,New Mexico, Mexico.
- Suleiman AKA, Lourenco KS, Clark C, Lima Luz R, da Silva GHR, et al. (2020) From toilet to agriculture: Fertilization with microalgal biomass from wastewater impacts the soil and rhizosphere active microbiomes, greenhouse gas emissions and plant growth. Resources Conservation and Recycling 161: 104924.
- Mahanty T, Bhattacharjee S, Goswami M, Bhattacharjee P, Tribedi P (2017) Biofertilizers: a potential approach for sustainable agriculture development. Environmental Science and Pollution Research 24(4): 3315-3335.
- Bidyarani N, Prasanna R, Babu S, Hossain F, Saxena AK (2016) Enhancement of plant growth and yields in Chickpea (Cicer arietinum L.) through novel cyanobacterial and biofilmed inoculants. Microbiological Research 188-189: 97-105.
- Ramakrishnan B, Kaur S, Prasanna R, Ranjan K, Kanchan A, Hossain F, et al. (2017) Microbial inoculation of seeds characteristically shapes the rhizosphere microbiome in desi and kabuli chickpea types. Journal of Soils and Sediments 17(8): 2040-2053.
- Algae Planet (2021) Myland soil-as-a-service reboots land’s natural potential. Environment.
- Shrestha RC, Ghazaryan L, Poodiack B, Zorin B, Gross A, Gillor O, et al. (2022) The effects of microalgae-based fertilization of wheat on yield, soil microbiome and nitrogen oxides emissions. Science of the Total Environment 806(Part 3): 151320.
© 2022 Liming Lai. This is an open access article distributed under the terms of the Creative Commons Attribution License , which permits unrestricted use, distribution, and build upon your work non-commercially.
 a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in
a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in 







.jpg)






























 Editorial Board Registrations
Editorial Board Registrations Submit your Article
Submit your Article Refer a Friend
Refer a Friend Advertise With Us
Advertise With Us
.jpg)






.jpg)














.bmp)
.jpg)
.png)
.jpg)










.jpg)






.png)

.png)



.png)






