- Submissions

Full Text
Modern Concepts & Developments in Agronomy
Application of Vacuum Infiltration for the Production of Haploid Plants of Cabbage Broccoli (Brassica oleracea L. Convar. Botrytis (L.) Alef. var cymosa Duch.)
SK Temirbekova1*, RN Kirakosyan2, EA Kalashnikova2, YUV Afanasyeva3, EV Maslova4, OV Meleshina1, and MM Tareeva5
1All-Russian Research Institute of Phytopathology, Russia
2Russian State Agrarian University-Timiryazev Moscow Agricultural Academy, Russia
3Federal Horticultural Research Center for Breeding, Agrotechnology and Nursery, Russia
4Belgorod State University, Russia
5Federal State Budgetary Scientific Institution Federal Scientific Vegetable Center, Russia
*Corresponding author: SK Temirbekova, All-Russian Research Institute of Phytopathology, Russia
Submission: February 01, 2022 Published: February 10, 2022

ISSN 2637-7659Volume10 Issue 2
Abstract
Relevance: One of the most important ways of classical breeding is creation of highly productive hybrids and varieties of agricultural plants. To speed up considerately the solution of this problem it is necessary to use in vitro technologies, in particular, production of haploid and diploid plants. Such technologies are of particular interest for growing important agricultural crops, for instance, broccoli. However, the frequency of plant regeneration from reproductive organs remains low. Therefore, nowadays it is necessary to search for high-performance and replicable technologies to produce DH plants.
Materials and methods: The objective of the work is to develop a procedure for producing plantsregenerants from the reproductive organs of broccoli (Brassica oleracea L. convar. Botrytis (L.) Alef. var cymosa Duch.) using the method of vacuum infiltration. The objects of the study were isolated anthers and ovaries of broccoli. The sort that was used is called “Dan` Nikolayu Vavilovu”. Vacuum infiltration of broccoli buds was carried out with a 15% solution of Aminoven at the concentration of 4ml/l and with a solution of Dropp at the concentration of 0.1mg/l. After the infiltration anthers and ovaries were isolated from the buds, which had been cultivated on MC nutrient medium containing the Dropp specimen at the concentration of 0.01mg/l and NAA 0.1mg/l.
Result: The use of vacuum infiltration of broccoli buds with Aminoven for 10 minutes along with pressure of 0.8 atm. was found to have a significant impact on embryogenesis process. Besides, this process took place directly on isolated anthers. In this case the frequency of embryoid formation averaged at about 3.7%. In the case of isolated ovaries usage, the frequency of embryogenesis was 2%. The proposed technology makes it possible to increase the frequency of the formation of haploid plants from isolated anthers and ovaries by 25-30% in comparison with the existing technologies of other authors
Keywords: Embryogenesis; Antheres; Ovaires; Broccoli sprout; Vacuum infiltration; In vitro
Introduction
Currently, the technology of obtaining haploids and doubled haploids is widely used in breeding programs to accelerate the development of high-yielding hybrids and varieties of agricultural plants [1]. This technology allows a necessary selection among the generations to achieve genetic stability and homogeneity of breeding lines [2], but it also reduces greatly the number of necessary populations to find the desired genotype.
DH technology has been improved over the last decades through empirical studies by different specialists working with a particular crop [3,4]. It can be further used in mutation breeding, genetic engineering, in vitro screening for complex traits such as drought, cold and salt tolerance. DH plants also play an integral role in the recently developed Reverse breeding technology. This technology involves recovering DHs from microspores of plants in which recombination is absent or limited (by suppressing or preventing recombination, e.g., by knocking out key meiotic genes). Produced recombinant-inbred populations can be screened using molecular markers to identify populations with complementary chromosome combinations to enable the reconstruction of the original heterozygous DH parent by hybridization of two individuals. Consequently, different parents with different chromosomal compositions can be identified to recover existing F1 hybrids.
Haploids (Hs) and doubled haploids (DH) can be produced either spontaneously or by different in vitro methods using female and male gametophytic cells/parts of the plant. For some monocotyledons and potatoes, both production options are possible, for most dicotyledons the current choice is limited to one method only [5].
It is well known that many basic protocols for producing haploids and doubled haploids were developed in the early 1990s, but most of them proved to be ineffective due to low reproducibility and low yield of DH-plants. This is due to the fact that the realization of the morphogenetic potential of the reproductive organs is determined by a complex of interrelated factors, such as genotype and growing conditions of the donor plant, pretreatment of buds and inflorescences, the stage of development of isolated anthers or microspores, the composition of the nutrient medium, etc. [6,7]. Each of these factors has its own specific influence on morphogenetic processes in vitro.
Particular attention in the development of regulations for obtaining haploid and dihaploid plants is paid to the hormonal control of morphogenesis, which is carried out by changing the auxin/cytokinin ratio. With the use of growth regulators, the interaction of cells, tissues and organs is carried out both in the whole plant and in its isolated explants. Hormones are necessary for triggering and regulating morphogenetic and physiological programs, they are active in very low concentrations and are relatively low-molecular weight substances. From the scholar data, it is known that when anthers are cultured on nutrient media in vitro, all plant species can be divided into two classes: hormonedependent and hormone-independent. In hormone-independent plants, hormones can either come from the anther wall or from the pollen itself. Hormone-independent plants are mainly in the Solanaceae family. For example, pollen embryogenesis was induced on simple mineral-sugar medium in tobacco [8], black henbane/ hog bean/poison tobacco/sticking Roger (Hyoscyamus niger) [9].
It has been found that tobacco anthers contain endogenous auxins, one of which was identified as indole-3-acetic acid (IAA) [10]. In order to induce callusogenesis and embryogenesis, anthers of most plant species require the addition of various hormones and other growth agents to the medium [11]. However, the substances under study do not always reach the target cells as they are not evenly distributed in the intercellular space. Therefore, a search for efficient technologies to deliver regulatory factors to culture in vitro plant cells is required. Such technology can be based on the application of the vacuum infiltration method.
As early as the 1930s, the vacuum infiltration method was applied to quantify the synthesizing and hydrolyzing action of invertase in living plant tissues. This method was based on introducing solutions of various substances into leaves by immersing the plant part under study in the solution, over which strong rarefaction of air was created. As a result, air left the intercellular spaces and then, under pressure, the solution got back into the leaves tissue. The rate and direction of enzymatic reactions in living cells were determined by this method [12].
Vacuum infiltration is used in modern technology, particularly in genetic engineering research for agrobacterial transformation. As a rule, the tissues used for transformation are immersed in a suspension of agrobacteria and then placed in a vacuum chamber for 20min or another time determined experimentally. Infiltration of plant tissues in a vacuum promotes the penetration of agrobacteria into the intercellular spaces. The work has been done on both somatic (leaf, petiole, meristem) and reproductive (bud) plant cells [13]. In 1999, for example, the method of an agrobacterial suspension vacuum infiltration directly into the flowers of intact Arabidopsis plants was proposed. The infiltration of the flowers was carried out for a few minutes. A subsequent return of the plants to soil conditions made it possible to obtain the transformed seeds. This technique was subsequently carried out on Chinese cabbage (Brassica chinensis, Brassica pekinensis), alfalfa (Medicago), colza (Brassica napus var. oleifera).
For example, in the works of Stepanova Ayu et al. [14] it is shown that an increase in transformation efficiency can be achieved if during incubation with agrobacteria wheat seeds are treated with ultrasound in the presence of aluminium oxide or if it is inoculation with vacuuming [14].
It is the principle of vacuum infiltration that can be used in in vitro technologies, and in particular in the production of haploid and diploid plants. Growth regulators, plant extracts and various preparations based on secondary plant metabolites can be used as inducers of morphogenesis. Probably, under the action of vacuum, the intercellular space can be filled with the studied solutions, which activate further morphogenetic processes. This method has not been developed for cell and tissue culture, and in particular for in vitro cultivation of reproductive organs of plants of the Brassicaceae family yet. Particular attention should be paid to broccoli cabbage.
Broccoli cabbage (Brassica oleracea L. convar. botrytis (L.) Alef. var cymosa Duch.) is common throughout the world due to its dietary, medicinal and preventive qualities and ease of cooking. In the Russian Federation, the recognized crops collection includes 50 varieties and hybrids of broccoli, of which 70% are of foreign selection and only 0.5% of the total production volume are native varieties [15].
Broccoli comes from the Mediterranean countries: Spain, Italy and Greece. In the last 10 years, the EU has begun to breed broccoli for higher cabbagehead weight and disease resistance intensively. The crop is high in protein, more than asparagus, spinach, sweet corn and yam. The curds contain essential amino acids, of which there are as many as in beef, and amounts of lysine, isoleucine and tryptophan are the same as, for example, in chicken eggs. In addition, broccoli cabbage curds contain methionine, choline, sulforaphane, sinigrin, vitamins A, B1, B2, PP, C, E, K, salts of potassium, magnesium, phosphorus, calcium, which improve memory, prevent the formation of cancer cells, enhance immunity, etc.
Because of the early maturity of this vegetable crop and its correct cultivation technique, it is possible to get several harvests per year. Considering that there are only two approved ultra-early maturing broccoli varieties and hybrids in the State Register of Breeding Achievements, it is necessary to fill this niche with native varieties. For this reason, conducting breeding work in the period from 2014 to 2019 allowed the team of researchers at Federal State Budgetary Scientific Institution “All-Russian Research Institute of Phytopathology”, led by doctor of biological sciences Temirbekova Sulukhan Kudaberdievna to produce a new variety of broccoli cabbage ‘Dan’ Nikolayu Vavilovu’, characterized by resistance to bacterial and fungal diseases, early maturity; with heads exceeding the standard weight and size (Figure 1). In December 2020, by the decision of the State Commission for Variety Testing and Protection of Breeding Achievements, this variety was included in the State Register for cultivation in all regions of the Russian Federation.
Regarding the study of broccoli cabbage in in vitro culture, in particular, the search and development of a highly efficient technology for producing haploid plants is still a topic of current interest. Based on the above, the purpose of the work is to develop regulations for obtaining broccoli cabbage (Brassica oleracea L. convar. botrytis (L.) Alef. var cymosa Duch.) plant regenerants from reproductive organs using the vacuum infiltration method.
Figure 1:
(a) Broccoli cabbage ‘Dan` Nikolayu Vavilovu’ in the phase of maturation and
(b) Flowering.

Material and Methods
The objects under study became isolated anthers and ovaries of broccoli cabbage. Research was conducted on the variety Dan’ Nikolayu Vavilovu. The plants were grown in a greenhouse at the Artificial Climate Laboratory (ACL) of Russian State Agrarian University-Timiryazev Moscow Agricultural Academy until the flowering stage.
The in vitro work was performed on buds, which were isolated from both leading and lateral shoots. Before introducing the isolated explants into the in vitro culture, a stepwise pretreatment was performed according to the method we developed earlier [16,17]. It includes the following stages: 1) incubation of buds in water for 1 day at low positive temperature (+4-6 °C); 2) after that, 2 days incubation in preparation Dropp solution (10mg/l); 3) sterilization of plant material in 0.1% mercury bichloride solution for 3-4 minutes, washing in three portions of sterile distilled water; following cultivation of isolated anthers or ovaries in vitro at +32 °C for 1 day on modified Murashige and Skoog (MS) nutrient medium [18].
Vacuum infiltration of sterile buds was performed before introducing anthers or ovaries into an in vitro culture. The work was performed in a vacuum chamber, in which sterile buds were placed and air was evacuated (0.7-0.9atm. pressure). After 40 minutes, the pressure was gradually increased to atmospheric conditions and the buds were incubated under these conditions for 1-15 minutes. The key point is that the difference in pressure causes the intercellular space to become filled with the test solution. The tested solutions were a 15% solution of Aminoven and Dropp at concentrations of 4ml/l and 0.1mg/l respectively. In the control version, the buds were subjected to vacuum infiltration with sterile distilled water, or vacuum infiltration was not applied, but the buds were kept in the prescribed solutions.
After vacuum infiltration, anthers or ovaries were isolated from buds under sterile conditions and transferred to nutrient media containing mineral salts according to MS prescription, and preparation Dropp at a concentration of 0.01mg/l, NAA 0.1mg/l. pH of the medium was in the range 5.5-5.8 in all variants. Plant material was cultured in Petri dishes in a climate chamber (Binder, Germany), in darkness at 350C for the first 5 days, after which temperature was reduced to 250C. Under these conditions, explants were grown for 25 days and then moved to a light room, where a 16-hour photoperiod and white luminescent lamps (brand “OSRAM AG”, production - Germany) with light intensity of 5 thousand lux were installed [16] (Kirakosyan, Kalashnikova 2015).
The experiments were carried out in triplicate. Statistical processing of the results was carried out according to standard methods. Data in the tables are given as arithmetic mean with standard error (M±mM). The differences of the chosen averages were assessed at a confidence level of 0.95.
Research Results
Based on the scholar literature data, we studied the effect of factors of chemical nature in vacuum infiltration conditions on the morphogenetic potential of cultivated reproductive organs of Brassica oleracea L. convar. botrytis (L.) Alef. var cymosa Duch.
Primarily, optimal time and pressure conditions should be established to provide high viability of cultured explants. Vacuum infiltration of the buds was performed for 1, 5, 10 and 15 min with water and at 0.7 atm, 0.8atm and 0.9atm pressure. The main results are shown in Figure 2.Figure 2: Influence of vacuum infiltration conditions on bud viability Brassica oleracea L. convar. botrytis (L.) Alef. var cymosa Duch.
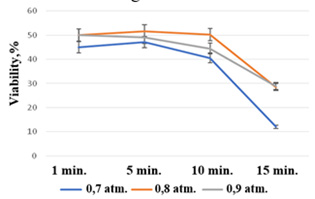
It has been shown experimentally that infiltration conditions have a significant effect on bud viability. An inversely proportional effect was found with increasing duration of vacuum infiltration buds viability decreased. This effect was shown by the formation of necrotic lesions primarily in the peripheral zone of buds during cultivation on nutrient medium MS combined with preparation Dropp and NAA at concentrations of 0.01 and 0.1mg/l respectively. Optimal vacuum infiltration conditions, which kept the buds viable in 50% of cases, were obtained at 0.8atm and with an exposure time of 1, 5 or 10 minutes. For next experiments, an exposure of 10 minutes was chosen for a longer impact of the chemicals on the explant, which is probably going to provide deeper permeation into the cellular structures (tissues).
Growth regulators and amino acids are known to be one of the important regulators of morphogenesis [17-19]. Therefore, searching ways to deliver these substances to the cells of cultivated explants is an actual problem. In this work the vacuum infiltration of broccoli cabbage buds with Dropp and a 15% solution of Aminoven was used. The substances were chosen on the basis of our previous studies, which proved their high stimulating action on the formation of adventive buds on cultured in vitro explants. For example, Aminoven stimulating action can be described by the content in the solution of 17 non-essential and essential L-amino acids (alanine, arginine, tyrosine, proline, glycine, etc.), its action on morphogenetic potential of somatic cells was proved in the experiments of R.G. Butenko (1999). The main results are given in Table 1&2.
Table 1: Influence of vacuum infiltration on the morphogenetic potential of isolated anthers of broccoli cabbage.
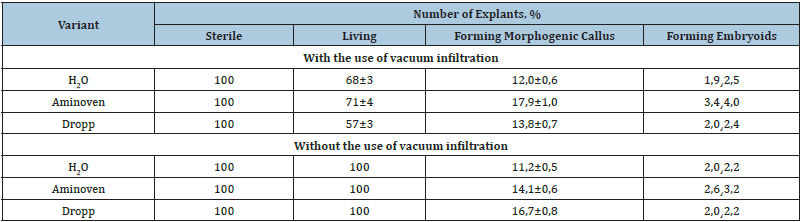
Table 2: Influence of vacuum infiltration on the morphogenetic potential of isolated broccoli cabbage ovaries.
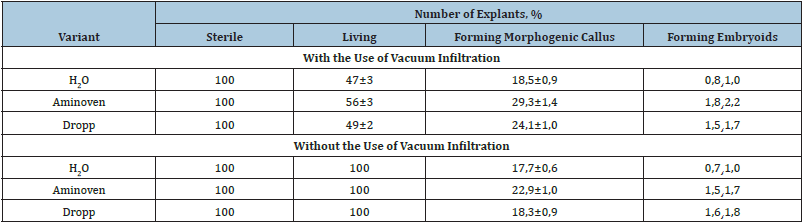
As a result of these studies, it is evident that the use of vacuum infiltration has a significant effect on the morphogenetic potential of cultured explants. It was experimentally shown that the action of vacuum could stimulate morphogenetic activity of cells, in relation to the control variant. Moreover, this effect depended on the studied explants [20,21]. For example, cultivation of anthers isolated from buds after vacuum infiltration led to a viability increase in explants and their morphogenetic potential. It should be also noted that the morphogenetic activity of explants was affected not only by vacuum infiltration, but also by the preparation that was used. Thus, when buds were infiltrated with a 15% solution of Aminoven, the process of embryogenesis was observed directly on isolated anthers. The frequency of embryogenesis in this variant was the highest, averaging 3.7%. Visual observations showed that the process of embryoid formation occurred asynchronously and all stages of embryoid development could be seen in the same field of view using a binocular loupe: globular, heart and torpedo (Figure 3).
Figure 3: Embryogenesis on isolated anthers of Brassica oleracea L. convar. botrytis (L.) Alef. var cymosa Duch.
after vacuum infiltration:
a. magnification x8,
b. magnification x12

As for cultivation of isolated ovaries, the rate of direct embryogenesis was between 0.8 and 2.2% for the variants. The highest rate was also obtained in the variant with the use of vacuum infiltration with Aminoven solution and the counted rate was 2.2%.
The formed embryoids from all variants were subsequently cultured on hormone-free MS nutrient medium on which the formation of seedlings was observed
It should be noted that during cultivation of isolated ovaries, in addition to direct plant regeneration, formation of callus tissue was found in 29.3% of cases. The following cultivation on MS nutrient medium containing IAA - 2mg/l, 6-BAP - 1mg/l, sucrose - 3%, agar - 7g/l resulted in the formation of adventitious buds, from which regenerated plants were subsequently formed (Figure 4).
Figure 4: Formation of callus tissue on isolated ovaries of Brassica oleracea L. convar. botrytis (L.) Alef. var cymosa Duch. after vacuum infiltration.

Conclusion
Thus, as a result of our research we have studied the dependence of reproductive organ morphogenesis process of broccoli cabbage on cultivation conditions. The positive effect of vacuum infiltration of cabbage buds for 10 minutes and 0.8atm. pressure, as well as the application of 15% solution of Aminoven drug on the process of morphogenesis was shown. The proposed technology makes it possible to increase the frequency of haploid plants formation from isolated anthers and ovaries by 25-30%, which is probably going to have an effect on speeding up the breeding process of deriving new hybrids and varieties of important agricultural plants.
References
- Ferrie AMR, Keller WA (2007) Optimization of methods for using polyethylene glycol as a non-permeating osmoticum for the induction of microspore embryogenesis in the Brassicaceae. In vitro Cell Dev Biol Plant 43(4): 348-355.
- Zhang HX, Hodson JN, Williams JP, Blumwald E (2001) Engineering salt tolerant Brassica plants: characterization of yield and seed oil quality in transgenic plants with increased vacuolar sodium accumulation. Proc Natl Acad Sci USA 98(22): 12832-12836.
- Charne DG, Pukacki P, Kott LS, Beversdorf WD (1988) Embryogenesis following cryopreservation in isolated microspores of rapeseed (Brassica napus). Plant Cell Rep 7(6): 407-409.
- Wang WC, Menon G, Hansen G (2003) Development of a novel Agrobacterium mediated transformation method to recover transgenic Brassica napus plants. Plant Cell Rep 22(4): 274-281.
- Touraev A, Forster BP, Jain SM (2009) Advances in haploid production in higher plants. The Netherlands, pp. 1-208.
- Dunwell JM (2010) Haploids in flowering plants: origins and exploitation. Plant Biotechnol J 8(4): 377-424.
- Zhang CG, Li W, Mao YF, Zhao DL (2005) Endogenous Hormonal levels in scutellaria baicalensis calli induced by thidiazuron. In: Zhang CG, Li W, Mao YF, Zhao DL, Dong W, et al. (Eds.), Russ J Plant Physiol 52(3): 345-351.
- Nitsch JP, Nitsch C (1969) Haploid plants from pollen grains. Science 163(3862): 85-87.
- Raghavan V (1976) Experimental eembryogenesis in vascular plants. Acad Press, London, UK, p. 603.
- Aionesei T, Touraev A, Heberle Bors E (2005) Pathways to microspore embryogenesis. Biotechnol Agr Forest 56: 11-34.
- Kalashnikova EA, May D (2010) Obtaining haploid plants of white cabbage in vitro. Potatoes and Vegetables 8: 26.
- Kursanov AL (1936) Application of the vacuum-infiltration method for quantitative determination of the synthesizing and hydrolyzing effects of invertases in living plant tissues. Biohimiya 1: 3.
- Xu H, Wang X, Zhao H, Liu F (2008) An intensive understanding of vacuum infiltration transformation of pakchoi (Brassica rapa Chinensis). Plant Cell Rep 27(8): 1369-1376.
- Stepanova A, Tereshonok DV, Osipova ES (2006) Obtaining transgenic wheat plants (T.a) by the method of agrobacterial transformation. Biotechnology 2: 20-27.
- Frolova O, Tareeva MM (2014) Broccoli. Taste and benefits from nature, Vegetables of Russia 4(25): 88-93.
- Kirakosyan RN, Kalashnikova EA (2015) Preparation of regenerating plants from the reproductive organs of white cabbage plants (Brassica oleracea ) in vitro. News of the Timiryazev Agricultural Academy 1: 18-25.
- Kirakosyan RN, Kalashnikova EA (2019) Cytological methods for the analysis of haploid regenerating plants of white cabbage (Brassica oleracea) obtained in vitro. Vegetables Crops of Russia 4(48): 13-15.
- Murashige TA, Skoog F (1962) Revised medium for rapid growth and bioassays with tobacco tissue cultures. Physiologia Plantarum 15(3): pp: 473-497.
- Yakimova OV, Egorova NA (2016) Influence of nutrient medium composition and genotype on clonal micropropagation of oregano in vitro. Proceedings of the Kuban State Agrarian University 4 (55): 304-309.
- Mohebalipour N (2012) Effect of plant growth regulators BAP and IAA on micropropagation of Iranian lemon balm (Melissa officinalis) landraces. In: Mohebalipour N, Aharizad S, Mohammadi SA (Eds.), Journal of Food Agriculture Environment 10(1): 280-286.
- Myagkih E (2018) Some features if vegetative propagation of Origanum vulgare In: Myagkih E, Yakimova O, Mishnev AV (Eds.), In vitro Cellular & Developmental Biology 54(4): 492.
© 2022 SK Temirbekova. This is an open access article distributed under the terms of the Creative Commons Attribution License , which permits unrestricted use, distribution, and build upon your work non-commercially.
 a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in
a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in 







.jpg)






























 Editorial Board Registrations
Editorial Board Registrations Submit your Article
Submit your Article Refer a Friend
Refer a Friend Advertise With Us
Advertise With Us
.jpg)






.jpg)














.bmp)
.jpg)
.png)
.jpg)










.jpg)






.png)

.png)



.png)






