- Submissions

Full Text
Modern Concepts & Developments in Agronomy
Effect of Curcumin Mediated Photodynamic Technology on Salmonella typhimurium Biofilm and Its Bactericidal Mechanism and Application in Milk
Dongli Dong1, Yimiao Deng1, Hui Li2, Wenqi Du1, Shuze Tang1*, Shaoling Lin3, Xiyang Wu4, Qingqing Zhou4, Charles Brennan5 and Zhenqiang Chen6
1Department of Food Science and Engineering, Jinan University, Guangzhou, China
2Guangdong Provincial Center for Disease Control and Prevention, Guanghou, China
3College of Food Science, Fujian Agriculture and Forestry University, Fuzhou, China
4China-New Zealand Joint Research Center for Food Safety & Nutrition, Jinan University, Guangzhou, China
5School of Science, RMIT University, GPO Box 2476, Melbourne 3001, Australia
6Department of Optoelectronic Engineering, Jinan University, Guangzhou, China
*Corresponding author: Shuze Tang, Department of Food Science and Engineering, Jinan University, Guangzhou, China
Submission: December 22, 2021 Published: January 10, 2022

ISSN 2637-7659Volume10 Issue 2
Abstract
The inactivation effect of curcumin-mediated photodynamic technology (PDT), a novel alternative nonthermal technique, on Salmonella typhimurium (S. typhimurium) biofilm and its preliminary bactericidal mechanism and application in milk were investigated. Biofilm formed from S. typhimurium ATCC 14028 was incubated with the photosensitizer curcumin, followed by exposure to blue laser (mad450nm) for testing antibiofilm effect. Planktonic S. typhimurium was taken for exploring the possible bactericidal mechanism. After curcumin-PDT treatment, the damages of bacterial DNA and protein were observed by agarose gel electrophoresis and sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE) respectively, and the morphology change was visualized by scanning electron microscopy (SEM). Confocal laser scanning microscopy (CLSM) was employed to test bacterial membrane permeability change. Bacterial and biofilm culture and curcumin-PDT preparation were conducted in a microbial laboratory, and during the PDT processing, all samples were illuminated in an optical laboratory. All experiments were completed in the Department of Food Science and Engineering of Jinan University, China from 2019 to 2020. Bacterial viability decreased significantly when curcumin concentration and illumination time increased. Curcumin (80μM) combined with blue light (200mW/cm2) illumination for 20min inactivated more than 2lg/(CFU/mL) S. typhimurium biofilm. DNA damage, protein degradation, and morphological change of PDT-treated S. typhimurium were observed. Curcumin-PDT treatment gained less bactericidal effect on S. typhimurium in milk and the inactivation efficacy was related to type of milk, curcumin concentration, illuminated liquid level and liquid transmittance. Therefore, curcumin-PDT is a promising method or assistance to control foodborne S. typhimurium in milk, but further investigations should be conducted to illustrate how the milk compositions interact with curcumin and light and how milk nutrients and sensory attributes response to curcumin-PDT treatment.
Keywords: Photodynamic technology; Curcumin; Salmonella typhimurium; Biofilm; Milk
Introduction
Rich in nutrients and welcomed by customers of all ages, milk also provides favorable
conditions for foodborne pathogens. It has a high risk of contamination by foodborne
pathogens such as Bacillus cereus [1], Cronobacter sakazakii [2] and Salmonella spp. [3]
Salmonella is one of the top-four foodborne pathogens leading to high morbidity and mortality [4,5]. Cases of 93.8 million gastroenteritis are caused by Salmonella
worldwide each year, resulting in nearly 155,000 deaths [6], and
S. typhimurium is one of the most common serotypes [7]. Reports
on milk in particular infant formula milk powder contaminated
by Salmonella always caused widespread social concern and
economic loss [8,9]. Besides bacteria existing in raw milk, bacteria
contaminating processing equipment can easily form biofilms then
contaminate products. Biofilm has been regarded as an intractable
problem in dairy industry, since the formation of biofilm will
increase the resistance of bacteria to adverse environment and
weaken the effect of disinfection [10]. Therefore, the control of
Salmonella biofilm that may contaminate food contact surfaces and
planktonic Salmonella in milk is of great importance during dairy
product processing.
Traditionally, thermal sterilization is the most commonly
employed method to inactivate foodborne pathogens, but it may
lead to the loss of heat-sensitive nutrients and the change of
organoleptic profiles [11]. Therefore, non-thermal processing
technologies such as disinfectant, high-pressure processing and
irradiation are considered to be potential for foodborne bacteria
disinfection. Recently, however, the shortcomings of these nonthermal
techniques were discussed. The problems of toxic residues,
high cost, professional requirement for operation and deterioration
of nutritional and organoleptic properties of food make it urgent to
find a novel alternative non-thermal technique to ensure the quality
and safety of milk product [12]. Photodynamic technology (PDT),
without above-mentioned disadvantages, is a promising technique
to prevent pathogens based on photochemical reactions [13,14].
In this process, the photosensitizer is activated by light at
specific wavelength and releases energy to form super-oxide,
hydroxyl radical or singlet oxygen which can react with adjacent
biological molecules to produce bacterial toxicity, leading to
damage or death of pathogens [15-17]. In recent years, increasing
PDT studies based on natural edible photosensitizers especially
curcumin have showed that PDT can inactivate a wide range of
foodborne bacteria with marginal damage to quality of various
food such as fruits, vegetables, meat and seafood products [18-
21]. Comparative research studies pointed out that S. typhimurium
can be effectively inactivated by PDT although it is more resistant
to this treatment as compared to other bacterial species [20,22-
24]. To our knowledge, no investigation has been carried out to
test curcumin-PDT effect on bacteria in milk and little literature is
available on curcumin-PDT to S. typhimurium biofilm. The aims of
this study, therefore, were to investigate curcumin-PDT inactivation
effect on S. typhimurium biofilm, explore preliminary inactivation
mechanism on planktonic S. typhimurium cells, and apply this
technique in milk.
Material and Methods
Bacterial culture preparation
S. typhimurium ATCC 14028 was gifted by Guangdong Provincial Center for Disease Prevention and Control (CDC), China. To obtain working culture, a single colony was picked and enriched in 5mL sterile tryptic soy broth (TSB) (Qingdao Hope Bio-Technology Co., Ltd, Qingdao, China), which was agitated at 37 ℃ and 120rpm for 16h. The planktonic cells were harvested by centrifugation at 3532×g for 10min, washed three times and re-suspended in phosphate-buffered saline (PBS) (109CFU/mL).
Photosensitizer and light source
Curcumin powder (>95%, Ci Yuan Biotechnology Co. LTD.,
Shanxi, China) was dissolved in ethanol (99%) as 20mM stock
solution which was then diluted with sterile water to obtain a
series of working concentrations. All the solutions were stored in
the dark at 4 ℃ before use. The ethanol concentration in working
solutions was <1% (v/v). It is worth pointing out that curcumin, as
a food additive, can be used in a range of 50-500mg/L or mg/kg in
different foods [23].
A blue 450nm laser (LWBL450-10W-F, Beijing Laser wave
Optoelectronics Technology Co., LTD, China) was used as the light
source and placed at 17cm distance above the sample. Energy
density on the surface of sample was (200mW/cm2) measured
with a Laser Power Meter (LI-P20W-A, Beijing Laser wave
Optoelectronics Technology Co., LTD, China).
PDT treatment on S. typhimurium biofilm
Every 200μL bacterial suspension and 5mL TSB were transferred to one well of 6-well plate. A microscope cover glass (20mm×20mm, 10212020C, Jiangsu, Citotest Scientific Co., Ltd, China) was put in each well and incubated at 30 ℃ for 48h to form biofilm. Then the TSB was removed, and the cover glass was washed with sterile PBS for three times. The biofilm on cover glass was incubated with a series of curcumin (0, 10, 20, 40, 80μM) in the dark at 37 ℃ for 5min. Then samples were illuminated at room temperature for 0, 1, 10, 20, 30 min, respectively. Experiments were divided into four groups: L-S- group (no light and curcumin), L-S+ group (curcumin alone), L+S- group (light alone) and L+S+ group (both light and curcumin). After illumination, the cover glass was placed in a centrifuge tube containing 5mL saline solution and treated by ultrasound for 10 min. One mL treated suspension was taken for 10-fold serial dilution followed by culturing on TSA plate for counting the viable bacteria. Each experiment was in triplicates.
Preliminary mechanism of bactericidal effect on planktonic S. typhimurium
Agarose gel electrophoresis analysis of S. typhimurium DNA: The planktonic cells were prepared as description in the section of bacterial culture preparation. Each aliquot (1.5mL) of bacterial suspension with 3.5mL curcumin (80μM) or PBS was mixed in one well of 6-well plate then illuminated for 30min. Treated samples were used for extracting bacterial DNA by bacterial total DNA extraction kit (Tian Gen Biochemical Technology Co. LTD., Beijing, China). Harvested DNA products were separated by 1.5% agarose gel electrophoresis, stained by golden view and visualized under UV light.
Sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE) analysis of S. typhimurium protein: Each aliquot (1.5mL) of bacterial suspension with 3.5mL curcumin (80μM) or PBS was mixed in one well of 6-well plate then illuminated for 30min. Each sample was collected (4mL) by centrifugation at 6149×g for 1min. The pellet was re-suspended in 20μL sterile water first and 20μL 2×SDS-gel loading buffer afterwards. After heating for 5 min at 95 ℃, the samples were centrifuged for another 2min. Ten μL supernatant was loaded and separated by 5% stacking gel and 12% separation gel electrophoresis (PowerPac™ Basic, Bio-Rad, America). Then the gel was stained by Coomassie Brilliant Blue.
Scanning electron microscopy (SEM) observation of S. typhimurium morphology
Each aliquot (1.5mL) of bacterial suspension with 3.5mL curcumin (80μM) or PBS was mixed in one well of 6-well plate and then illuminated for 30min. Each sample was collected (4mL) by centrifugation at 3532×g for 10min and washed with PBS for three times. The pellet was fixed in 1mL glutaraldehyde (2.5%) for 12h at 4 ℃ then dehydrated in ethanol solutions of graded series concentrations (once at 30, 50, 70, 80 and 90%, and twice at 100%) before critical point drying in carbon dioxide. Finally, samples were coated with gold and observed under SEM (Supra55, Zeiss, Germany).
Confocal laser scanning microscope observation of S. typhimurium membrane permeability: Change of cell permeability after PDT treatment was measured by LIVE/DEAD® BacLight™ bacterial viability kit (Invitrogen, Thermo Fisher Scientific, America). Biofilm was formed on the cover glass and exposed to 80μM curcumin combined 30-min light illumination treatment (L+P+) or untreated (L-P-). Subsequently, the glass was picked up and washed for three times by sterile water. Mixture (150μL) of SYTO9 and propidium iodide (PI) was added to cover the sample on glass surface, followed by a 15-min dark incubation at 37 ℃ and washing for three times. Then samples were added a drop of anti-fluorescence quenching solution respectively, prior to their observation by confocal laser scanning microscope (CLSM, LSM880, Zeiss, Germany).
PDT treatment on planktonic S. typhimurium in milk
Table 1: Instruction of milk solution preparation.

*BS is base saturation
Infant formula (Friso, Friesland Campina (Hong Kong) Limited,
China), instant milk powder (Devondale, Murray Goulburn Co-
Operative Co. Limited, Australia) and instant skim milk powder
(Devondale, Murray Goulburn Co-Operative Co. Limited, Australia)
were purchased from local supermarket in Guangzhou. Different
types of milk solutions were prepared according to instructions
given by manufacturers (Table 1). Curcumin stock solution (0.10mL,
20mM) compounded with 24.90mL and 16.57mL milk solutions
respectively to gain 80μM and 120μM turmeric milk.
To test the effect of curcumin concentration on bactericidal
efficacy, 1mL bacterial aliquot was mixed with the equal volume
of PBS, 80μM and 120μM turmeric milk solutions respectively in
a 6-well plate. One mL or 2mL bacterial aliquot was mixed with
the equal volume of PBS and 120μM turmeric milk solutions
respectively to test the effect of liquid level (0.2cm or 0.4cm) on
bactericidal efficacy. Then the turmeric milk solutions were diluted
to gain double dilution milk, and 1mL bacterial aliquot was mixed
with the equal volume of PBS, 120μM raw turmeric milk and 120μM
double diluted turmeric milk solutions respectively to test the effect
of milk dilution on bactericidal efficacy. All plates after incubation
in the dark at 37 ℃ for 5 min, were placed under the blue light to
illuminate for 30min. One mL treated sample was then taken for
10-fold serial dilution, and 100μL of each dilution was spread on
TSA plate and incubated at 37 ℃ for 16h for counting viable cells.
The infant formula milk solution was diluted to gain twofold
and tenfold dilution milk for testing light transmittance. The
light transmittance of milk samples was measured by UV
spectrophotometer. Each experiment was in triplicates.
Statistical analysis
All data are expressed as mean ± standard deviation (SD) from three separate experiments. Statistical analysis was performed by one-way analysis of variance (ANOVA) using Origin 9.0 software.
Result and Discussion
PDT inactivation of S. typhimurium biofilm
Effect of exposure time: The effect of illumination time on PDT inactivation of S. typhimurium biofilm was shown in Figure 1. Illumination alone did not affect the S. typhimurium biofilm viability obviously, even the illumination time was prolonged to 30min. Curcumin (20μM) combined with blue light had the inactivation effect on S. typhimurium biofilm, and 1.9lg/(CFU/mL) viability decrease was obtained after 30 min PDT treatment.
Figure 1: Effect of illumination time on PDT inactivation of S. typhimurium biofilm. Different upper letters and lowercase letters represent significant difference in S- group (without curcumin) and S+ (with curcumin, 20μM) group respectively.

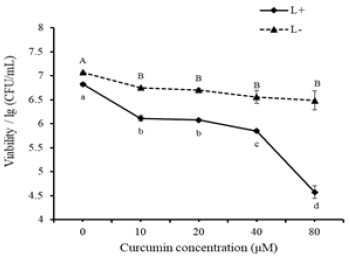
Effect of curcumin concentration: Data in Figure 2 demonstrated that curcumin had a mild bactericidal effect by itself. Eighty μM curcumin could reduce approximate 0.6lg/(CFU/mL) biofilm viability. When combined with blue light, curcumin showed more significant inactivation effect on S. typhimurium biofilm. The inactivation effect was strengthened when curcumin concentration increased, and 80μM curcumin reduced the most biofilm viability (more than 2lg/(CFU/mL)) after 20-min illumination. A previous study reported that curcumin treatment alone caused cell membrane damage and cell death because the insertion of curcumin into the liposome bilayer enhanced cell membrane permeability [25,26], but it took longer time to achieve the same bactericidal effect as PDT. Previous studies pointed out that grampositive bacteria were more susceptible than gram-negative bacteria [23,24] and bacterial biofilm inactivation by PDT was more difficult than planktonic bacteria [27,28]. Present viability results suggested that curcumin-PDT could penetrate extracellular polymeric substance (EPS) and injured bacteria inside. Penha et al. [23] examined the bactericidal effect of 75μM curcumin and 30 min blue LED (λmax 470nm) illumination, showing a 2.82log CFU/mL reduction of S. typhimurium. This treatment conditions and results were quite similar to that in present study, indicating that curcumin-PDT can also be a potential strategy for controlling both planktonic S. typhimurium and its biofilm. Bonin et al. [24] exposed S. typhimurium to the combined treatment of 10μM eosin Y and 15-min green LED (λmax 490-570nm) illumination, causing a 1.7 log CFU/mL reduction. Santos et al. [22] compared PDT effects mediated by eosin and rose bengal respectively on S. typhimurium, and 100μM eosin combined 15-min illumination (530±40nm) could only lead to about 2 log reduction. In contrast, 50μM rose bengal with 15-min illumination (530±40nm) led to the total inactivation. Although, some photosensitizers in PDT treatment could result in very desirable inactivation effects, they are synthesized dyes and not suitable for food application.
Figure 2: Effect of curcumin concentration on PDT inactivation of S. typhimurium biofilm. Different upper letters and lowercase letters represent significant difference in L- (without light) group and L+ (with light, 20min) group respectively.
Preliminary mechanism of PDT inactivation of S. typhimurium biofilm
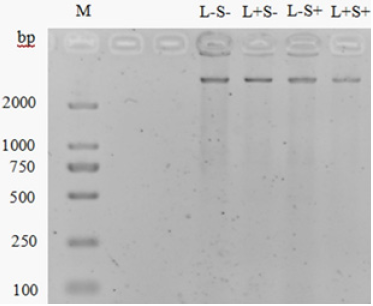
Damage of bacterial DNA: In Figure 3, the intensity of DNA band in L-S+ group showed a marginal decrease compared with bands in L+P+ and L+P- groups, indicating that light alone could not damage bacterial DNA but 80μM curcumin treatment alone might lead to a slight DNA damage. The DNA band in L+S+ group was the weakest, showing the most significant damage compared with other groups, but still not disappeared completely. It is consistent with the DNA damage of Vibrio parahaemolyticus by MB-PDT reported by Deng et al. [29]. Other studies have shown that PDT enhanced the control of pathogens by attenuating quorum sensing (QS) dependent factors, such as production of exopolysaccharide and alginate, swimming ability and virulence factors [30-32]. However, this study only showed the PDT effect on total bacterial DNA by SDS-PAGE for a preliminary conclusion. The quantification of gene damage and analyzing specific gene damage caused by PDT can be further carried out by real-time polymerase.
Figure 3: Agarose gel electrophoresis analysis of S. typhimurium DNA. M: Marker, DL 2000. L-S-: no light and curcumin. L+S-: light alone (30min). L-S+: curcumin alone (80μM). L+S+: both light (30min) and curcumin (80μM).
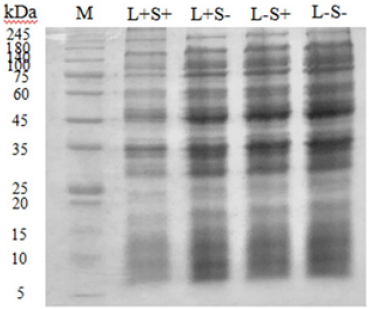
Damage of bacterial protein: In Figure 4, protein degradation of S. typhimurium was analyzed by SDS-PAGE. Intensity of protein bands in L-P- (control), L-P+ (only curcumin), L+P- (only light) groups did not change obviously, showing that compared with control group, neither individual curcumin nor only light treatment could affect S. typhimurium protein. In contrast, slight decreasing intensity of protein band was observed in L+P+ group, suggesting that bacterial protein degradation might be caused by 80μM curcumin in combination with 30 min illumination treatment. Results obtained in this study proved curcumin-PDT caused damage of S. typhimurium total protein. Similarly, Li et al. [21] observed a slight degradation of total Salmonella spp. protein by riboflavinmediated PDT. Wu et al. [33] showed that PDT damaged bacterial outer membrane protein. But hardly any report has further studied other PDT-targeted proteins of bacteria. Actually, the oxidative burst of reactive oxygen species (ROS) produced by PDT will break the balance of microorganism homeostasis and attack those substances playing important physiological roles in cells such as RNA and lipid, besides DNA and protein [34].
Figure 4: SDS-PAGE analysis of S. typhimurium protein. M: Marker (5-245kDa). L-S-: no light and curcumin. L+S-: light alone (30min). L-S+: curcumin alone (80μM). L+S+: both light (30min) and curcumin (80μM).
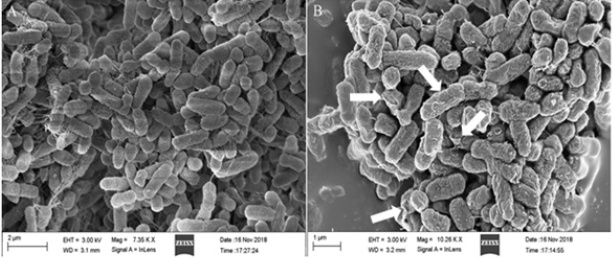
Change of bacterial morphology: Morphological change of S. typhimurium after PDT treatment was shown in Figure 5. The bacteria of L-S- group were oval and plump, while bacterial cells in L+S+ group were wrinkled and irregular, even some cracks occurred on the surface (see arrows in Figure 5B). This result visually showed that curcumin-PDT distorted morphology of S. typhimurium but did not cause collapse of cells. Chai et al. [35] observed no significant change of L. monocytogenes morphology after curcumin-PDT by SEM, but further transmission electron microscopy (TEM) observation found partial cytoplasm cavitation. Therefore, the main target of PDT might be the disruption of intracellular biological damage.
Figure 5: SEM images of S. typhimurium. A: L-S-, no light and curcumin. B: L+S+, both light (30min) and curcumin (80μM).
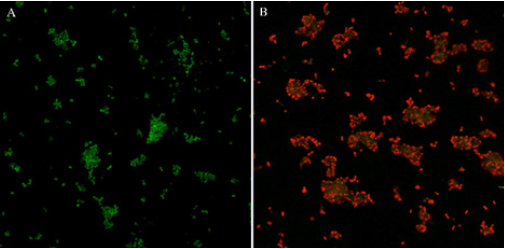
Change of membrane permeability: Since membrane integrity is indispensable for bacterial survival, based on the different transmembrane ability of SYTO 9 and PI, live and dead cells can be distinguished by double stains SYTO 9 and PI. SYTO 9 can cross cell membrane and label all bacteria with green fluorescence when used alone while PI can only penetrate cells when membrane injured or disrupted and present red fluorescence [36]. CLSM was used to observe the permeability of bacteria in biofilms after PDT treatment of 80μM curcumin in combination with 30-min illumination. As shown in Figure 6, cells in L-S- group were green, showing bacterial membrane remaining intact and blocked PI stain outside the cells. In contrast, most cells in L+S+ group presented red, indicating PDT treatment caused membrane permeability damage and PI can went inside. Therefore, the result suggested that curcumin-PDT could penetrate or disrupted bacterial EPS and induced S. typhimurium permeability damage, leading to lethal injuries of inside bacteria. However, how PDT interacted and affected protective bacterial EPS could not be explained in present study. Further study on PDT effect on EPS in detail needs to be carried out.
Figure 6: Live/dead fluorescent staining images of S. typhimurium. A: L-S-, no light and curcumin. B: L+S+, both light (30 min) and curcumin (80μM).
PDT inactivation of S. typhimurium in milk
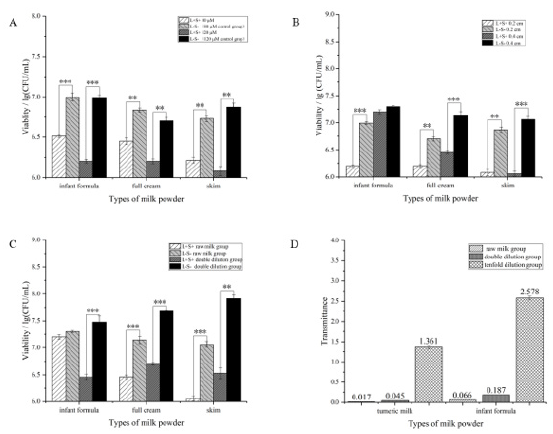
Results in Figure 7 showed that when PDT was applied in milk,
type of milk, curcumin concentration, illuminated liquid level and
liquid transmittance all affected inactivation efficacy. In Figure 7A,
PDT inactivation effect on S. typhimurium in full cream milk was
the weakest in terms of three types of milk. Increasing curcumin
concentration to 120μM could decrease milk-borne S. typhimurium
in full cream milk by around 0.5lg/(CFU/mL) while about 0.8lg/
(CFU/mL) decline was observed in other two kinds of milk. The
difference might be attributed to the lipid content in milk powder.
Since the external structural integrity of gram-negative bacteria
is closely related to lipopolysaccharide (LPS), the higher level of
lipid content in milk may be more conducive to maintaining the
lipid soluble structure of bacteria. Undesirable bactericidal effect
in milk was also observed by Wang et al. [37] Staphylococcus
aureus in milk was just reduced by 0.42 log after Na-chlorophyllin-
PDT treatment, while 4.5 log bacteria could be reduced in saline
solution. Such a significant difference was presumed to be caused
by large solid particles in milk which sheltered and returned light
to block PDT reaction. Galstyan [38] considered that reduction
of bactericidal effect in milk was due to aggregation effect of
casein and whey protein on photosensitizer, which changed the
maximum absorption spectrum of photosensitizer. The existence of
cysteine could also quench the singlet oxygen produced during the
photodynamic process. In addition, calcium and magnesium ions in
the emulsion could stabilize the negative charge in oligosaccharide
chains and might seriously affect the binding of photosensitizer to
gram-negative bacteria.
Figure 7B showed that the PDT effect did not change rapidly
when the liquid level of full cream and skim milk changed, while the
bactericidal effect on infant formula milk was totally inhibited when
the illuminated liquid level changed from 0.2cm to 0.4cm. It might
be that the infant formula milk contained more trace elements and
minerals (iron, zinc, vitamins), so blue light could not penetrate the
emulsion effectively, and the light intensity reaching the surface
and bottom of the sample was unequal, which greatly reduced the
bactericidal effect.
Besides, the bactericidal effect was significantly enhanced
with the increase of milk dilution ratio (Figure 7C). Especially for
skim milk, S. typhimurium reduction in double diluted milk was
the most, reaching almost 1.4lg/(CFU/mL). Figure 7D revealed
that transmittance of milk increased with the increase of dilution
ratio, indicating the positive correlation between PDT efficacy and
solution transmittance. Wang et al. [37] also proved it by comparing
the PDT inactivation effect on S. aureus in clear and cloudy litchi
juice. Therefore, application of PDT in cloudy liquid food will be
more difficult.
Figure 7: Factors involved in PDT effect on S. typhimurium in milk.
A. Curcumin concentration (80μM+0.2 cm; 120μM+0.2cm).
B. Liquid level (120μM+0.2cm; 120μM+0.4cm).
C. Milk dilution ratio (raw milk; double diluted milk).
D. Light transmittance of milk with different dilution ratio.
Conclusion
This study showed that curcumin in combination with blue
light could inactivate S. typhimurium biofilm by more than 2lg. For
planktonic S. typhimurium, curcumin-PDT could cause genomic
DNA damage and protein degradation, and distorted S. typhimurium
morphology, induced membrane damage and permeability change,
leading to S. typhimurium cell death. When curcumin-PDT was
adopted to control S. typhimurium in milk, type of milk, curcumin
concentration, illuminated liquid level and liquid transmittance
all affected PDT efficacy. Curcumin (120μM) in combination with
illumination (30min) obtained the best bactericidal effect in double
diluted skim milk with a liquid level of 0.2cm.
Even though inactivation effects of curcumin-PDT were observed
on both S. typhimurium biofilm in vitro and planktic S. typhimurium
cells in milk, further investigations are necessary to be implemented.
Firstly, the curcumin-PDT inactivation of S. typhimurium biofilm in
food or food contact surfaces should be carried out. Secondly, it is
important to figure out the interactions between milk compositions
(such as protein and fat) and curcumin and light, and the attributes
change of milk before and after treatment. Thirdly, since its limited
penetrability in cloudy solution, the possibility of combination with
other bactericidal technologies also deserves future investigation.
Furthermore, comparative studies of curcumin-PDT in various
products and foodborne pathogens can be valuable and helpful to
identify its accurate application.
Acknowledgement
The authors are grateful to the National Key Research and Development Project (2018YFG1602504), China-New Zealand Joint Research Center for Food Safety & Nutrition (20200502) and Fujian Provincial Collaboration Project (2020I0010) for their financial supports.
References
- Di Pinto A, Bonerba E, Bozzo G, Ceci E, Terio V, et al. (2013) Occurrence of potentially enterotoxigenic Bacillus cereus in infant milk powder. Eur Food Res Technol 237(2): 275-279.
- Henry M, Fouladkhah A (2019) Outbreak history, biofilm formation, and preventive measures for control of Cronobacter sakazakii in infant formula and infant care settings. Microorganisms 7(3): 77-77.
- Hayman MM, Edelson Mammel SG, Carter PJ, Chen YI, Metz M, et al. (2020) Prevalence of Cronobacter spp. and Salmonella in milk powder manufacturing facilities in the United States. J Food Prot 83(10): 1685-1692.
- Antunes P, Mourão J, Campos J, Peixe L (2016) Salmonellosis: the role of poultry meat. Clin Microbiol Infect 22(2): 110-121.
- Eng SK, Pusparajah P, Ab Mutalib NS, Ser HL, Lee LH (2015) Salmonella: a review on pathogenesis, epidemiology and antibiotic resistance. Front Life Sci 8(3): 284-293.
- Majowicz SE, Musto J, Scallan E, Angulo FJ, Kirk M, et al. (2010) The global burden of nontyphoidal Salmonella gastroenteritis. Clin Infect Dis 50(6): 882-889.
- Alcaine SD, Warnick LD, Wiedmann M (2007) Antimicrobial resistance in nontyphoidal Salmonella. J Food Prot 70(3): 780-790.
- Jones G, de la Gandara MP, Herrera Leon L, Herrera Leon S, Martinez CV, et al. (2019) Outbreak of Salmonella enterica serotype Poona in infants linked to persistent Salmonella contamination in an infant formula manufacturing facility, France, August 2018 to February 2019. Euro Surveill 24(13): 1900161.
- Rodriguez Urrego J, Herrera Leon S, Echeita Sarriondia A, Soler P, Simon F, et al. (2010) Nationwide outbreak of Salmonella serotype Kedougou associated with infant formula, Spain, 2008. Euro Surveill 15(22): 19582.
- Simões M, Simões LC, Vieira MJ (2010) A review of current and emergent biofilm control strategies. LWT-Food Sci Technol 43(4): 573-583.
- Coutinho NM, Silveira MR, Rocha RS, Moraes J, Ferreira MVS, et al. (2018) Cold plasma processing of milk and dairy products. Trends Food Sci Technol 74: 56-68.
- Meireles A, Giaouris E, Simões M (2016) Alternative disinfection methods to chlorine for use in the fresh-cut industry. Food Res Int 82: 71-85.
- Castano AP, Demidova TN, Hamblin MR (2004) Mechanisms in photodynamic therapy: part one-photosensitizers, photochemistry and cellular localization. Photodiagnosis Photodyn Ther 1(4): 279-293.
- Hamblin MR, Hasan T (2004) Photodynamic therapy: a new antimicrobial approach to infectious disease? Photochem Photobiol Sci 3(5): 436-450.
- Abrahamse H, Hamblin MR (2016) New photosensitizers for photodynamic therapy. Biochem J 473(4): 347-364.
- Insińska Rak M, Sikorski M (2014) Riboflavin interactions with oxygen-a survey from the photochemical perspective. Chemistry 20(47): 15280-15291.
- Wozniak A, Grinholc M (2018) Combined antimicrobial activity of photodynamic inactivation and antimicrobials-state of the art. Front Microbiol 9: 930.
- Lin Y, Hu J, Li S, Hamzah SS, Jiang H, et al. (2019) Curcumin-based photodynamic sterilization for preservation of fresh-cut Hami melon. Molecules 24(13): 2374.
- Glueck M, Schamberger B, Eckl P, Plaetzer K (2017) New horizons in microbiological food safety: Photodynamic Decontamination based on a curcumin derivative. Photochem Photobiol Sci 16(12): 1784-1791.
- Gao J, Matthews KR (2020) Effects of the photosensitizer curcumin in inactivating foodborne pathogens. Food Control 109: 106959.
- Li H, Tan L, Chen B, Huang J, Zeng Q, et al. (2021) Antibacterial potency of riboflavin-mediated photodynamic inactivation against Salmonella and its influences on tuna quality. LWT 146:
- Santos AR, Batista A, Gomes A, Neves M, Faustino M, et al. (2019) The remarkable effect of potassium iodide in eosin and rose bengal photodynamic action against Salmonella typhimurium and Staphylococcus aureus. Antibiotics 8(4): 211.
- Penha CB, Bonin E, da Silva AF, Hioka N, Zanqueta EB, et al. (2017) Photodynamic inactivation of foodborne and food spoilage bacteria by curcumin. LWT-Food Sci Technol 76: 198-202.
- Bonin E, Dos Santos AR, Fiori da Silva A, Ribeiro LH, Favero ME, et al. (2018) Photodynamic inactivation of foodborne bacteria by eosin Y. J Appl Microbiol 124(6): 1617-1628.
- Tyagi P, Singh M, Kumari H, Kumari A, Mukhopadhyay K (2015) Bactericidal activity of curcumin I is associated with damaging of bacterial membrane. PloS one 10(3): e0121313.
- Varshney GK, Saini RK, Gupta PK, Das K (2013) Effect of curcumin on the diffusion kinetics of a hemicyanine dye, LDS-698, across a lipid bilayer probed by second harmonic spectroscopy. Langmuir 29(9): 2912-2918.
- Andrade MC, Ribeiro AP, Dovigo LN, Brunetti IL, Giampaolo ET (2013) Effect of different pre-irradiation times on curcumin-mediated photodynamic therapy against planktonic cultures and biofilms of Candida spp. Arch Oral Biol 58(2): 200-210.
- Street CN, Gibbs A, Pedigo L, Andersen D, Loebel NG (2009) In vitro photodynamic eradication of Pseudomonas aeruginosa in planktonic and biofilm culture. Photochem Photobiol 85(1): 137-143.
- Deng X, Tang SZ, Wu Q, Tian J, Riley WW, et al. (2016) Inactivation of Vibrio parahaemolyticus by antimicrobial photodynamic technology using methylene blue. J Sci Food Agric 96(5): 1601-1608.
- Packiavathy IA, Priya S, Pandian SK, Ravi AV (2014) Inhibition of biofilm development of uropathogens by curcumin - an anti-quorum sensing agent from Curcuma longa. Food Chem 148: 453-460.
- Wang Y, Lu Z, Wu H, Lv F (2009) Study on the antibiotic activity of microcapsule curcumin against foodborne pathogens. Int J Food Microbiol 136(1): 71-74.
- Zhou X, Zhang B, Cui Y, Chen S, Teng Z, et al. (2017) Curcumin promotes the clearance of Listeria monocytogenes both in vitro and in vivo by reducing listeriolysin o oligomers. Front Immunol 8: 574.
- Wu J, Mou H, Xue C, Leung AW, Xu C, et al. (2016) Photodynamic effect of curcumin on Vibrio parahaemolyticus. Photodiagnosis Photodyn Ther 15: 34-39.
- Lam PL, Wong RS, Lam KH, Hung LK, Wong MM, et al. (2020) The role of reactive oxygen species in the biological activity of antimicrobial agents: an updated mini review. Chem Biol Interact 320: 109023.
- Chai Z, Zhang F, Liu B, Chen X, Meng X (2021) Antibacterial mechanism and preservation effect of curcumin-based photodynamic extends the shelf life of fresh-cut pears. LWT 142: 110941.
- Robertson J, McGoverin C, Vanholsbeeck F, Swift S (2019) Optimisation of the protocol for the LIVE/DEAD® BacLight TM bacterial viability kit for rapid determination of bacterial load. Front Microbiol 10: 801.
- Wang XQ, Liu ZY, Yu YS, Xu YJ, Wu JJ., et al. (2013) Photodynamic sterilization of Staphylococcus aureus in liquid food by Na-chlorophyllin. Mod Food Sci Technol 29(3): 463-466.
- Galstyan A, Dobrindt U (2019) Determining and unravelling origins of reduced photoinactivation efficacy of bacteria in milk. J Photochem Photobiol B 197: 111554.
© 2021 Shuze Tang. This is an open access article distributed under the terms of the Creative Commons Attribution License , which permits unrestricted use, distribution, and build upon your work non-commercially.
 a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in
a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in 







.jpg)






























 Editorial Board Registrations
Editorial Board Registrations Submit your Article
Submit your Article Refer a Friend
Refer a Friend Advertise With Us
Advertise With Us
.jpg)






.jpg)














.bmp)
.jpg)
.png)
.jpg)










.jpg)






.png)

.png)



.png)






