- Submissions

Full Text
Modern Concepts & Developments in Agronomy
The Close Relationship between the Careless Production of New Apricot Trees and the Spread of a Causal Agent of Bacterial Canker in Apricot Orchards
Iveta Pánková*, Václav Krejzar and Radka Krejzarová
Crop Research Institute, Drnovská 507, 16106 Praha 6, Czech Republic
*Corresponding author: Iveta Pánková, Crop Research Institute, Drnovská 507, 16106 Praha 6, Czech Republic
Submission: November 19, 2021Published: December 07, 2021

ISSN 2637-7659Volume9 Issue 5
Abstract
Bacterial canker and the premature death of young stone fruit trees, caused by different members of the Pseudomonas syringae (Ps) complex, affects commercially grown apricot orchards. Altogether, 70% of samples of mother apricot tree scion varieties (Prunus armeniaca L.) from different European localities consisted of Pseudomonas strains which are highly pathogenic to detached apricot twigs in the pathogenicity test. These strains were attributed to phylogroup PG02 and PG03, and rpoD sequencing confirmed a similarity to strains of Pseudomonas syringae pv. syringae and Pseudomonas amygdali pv. morsprunorum race 1 known to be pathogenic to apricot, respectively.
Keywords: Scion, Pseudomonas amygdali pv. Morsprunorum, Pseudomonas syringae pv. syringae
Introduction
For the production of apricot trees, only certified scions of apricot cultivars should be used. Propagating fruit materials are compulsorily tested for the presence of quarantine pests. Although apricot tree losses of up to 80% due to the bacterial canker and premature death have been reported all over the world, scion materials are rarely tested for the presence of disease causal agents.
Methodology
Screening of the causal agents of bacterial canker in mother apricot trees of scion varieties from 8 European localities was carried out in 2019-2021. The internal tissues of 30 buds from each scion variety were analysed using culture-dependent methods. Based on the colony morphology, Pseudomonas-like colonies from each sample were cultured on King’s B medium and evaluated in a hypersensitive reaction test on tobacco and for ice nucleation activity and determined by the FAME method [1-3]. A subset of strains determined by FAME as belonging to the genus Pseudomonas with a similarity index of SimIndex≥0.5 and/or strains with positive HR and INA within (0 °C; -6 °C) were attributed to the phylogroups of Pseudomonas syringae (Ps) complex and their pathogenicity on detached apricot twigs was evaluated [4,5]. The strains representing all PGs were selected for phylogenetic characterization based on partial sequences of the housekeeping gene rpoD [6]. The results of the screening of apricot scion varieties from different localities and different samples of propagating materials were subjected to ANOVA.
The strict use of healthy propagating materials as the most important preventive measures against the bacterial canker of apricot
Knowledge of various reservoirs of Pseudomonas bacteria and their traits relative to the aptitude to survive and spread in apricot orchards is particularly pertinent for implementing preventive measures [7]. The results of this survey displayed the necessity to screen all propagating materials of apricot for the causal agent of bacterial canker, and to improve management practices in scion mother orchards. Varying weather conditions in the nurseries where mother apricot trees of scion varieties are grown and the locality where new apricot trees are produced and planted reveal hidden infection and the effect development of bacterial canker disease. Large scale dissemination can occur when apparently healthy but latently infected propagating materials are introduced. Ps strains maintain a high level of adaptability, both as a symptomless member of epiphytic populations on the leaf surface and as an endophytic pathogen [8,9]. Within epiphytic microflora, a different size of pathogenic populations which are able to move towards woody plant tissues that provide nutrients and protected niches [10,11] during the dormant period is established according to the agricultural measurements, environmental and seasonal weather conditions. Bacterial control in mother apricot nurseries is the way how to reduce the dramatically premature death of apricot trees.
Result
Given the age of dying saplings between 2-5-years-old and the absence of severe external symptoms of the bacterial canker disease, the possibility of the presence of systemic infection in apricot orchards was considered. The pathogens can be transmitted by bud grafting and, ultimately even the small size of the pathogen population results in 20-50 % losses of trees in new apricot plantings within three years. The occurrence of causal agents of premature death in mother apricot trees scion varieties grown in the nurseries in eight European localities was screened in 2019- 2021 (Figure 1).
Figure 1: The incidence of strains from the Pseudomonas syringae complex, with an emphasis on strains pathogenic to apricot and ice nucleation active strains in mother apricot trees scion varieties grown in the nurseries in eight European localities in 2019-2021.
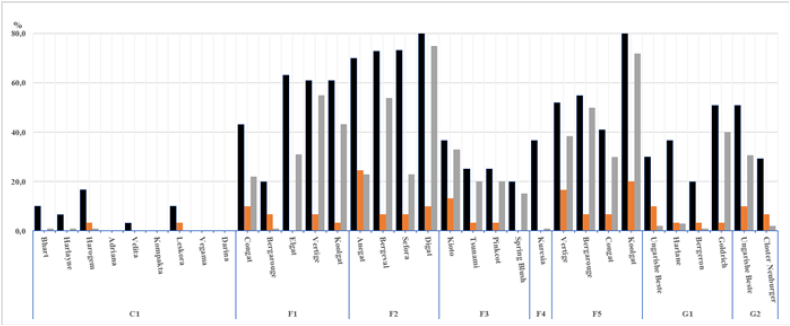
Values of p ≤ 0.05 (0.01; 0.05) showed the significant effect of the locality on the incidence of strains from Ps complex (black bars), Ps strains positive in ice nucleation activity (yellow bars) and strains pathogenic to detached apricot twigs (grey bars). The threeyear survey revealed that the most aggressive strains, accounting for 5% of Pseudomonas strains, were attributed to the phylogroup PG03 within the Ps complex and their rpoD sequences showed a high similarity to sequences of the PG03 reference strain P. amygdali pv. morsprunorum race 1 FTRS_U7805. Regardless of the locality and scion variety, the most abundant were Ps strains attributed to the phylogroup PG02 (44%), slightly pathogenic to apricot, positive in ice nucleation activity and showed a high similarity (≥99%) to rpoD partial sequences of Ps pv. syringae strains.
Acknowledgement
This study was supported by the National Agency for Agricultural Research of the Czech Republic under the project QK1920058 and the Ministry of Agriculture of the Czech Republic within the project RO0418.
References
- Klement Z, Goodman RN (1967) Role of living bacterial cell and induction time in hypersensitive reaction of tobacco plant. Phytopathology 57(3): 322.
- Lindow SE, Arny DC, Upper CD (1982) Bacterial ice nucleation: a factor in frost injury to plants. Plant Physiology 70(4): 1084-1089.
- Borschinger B, Bartoli C, Chandeysson C, Guilbaud C, Parisi L, et al. (2016) A set of PCRs for rapid identification and characterization of Pseudomonas syringae Journal of Applied Microbiology 120(3): 714-723.
- Parisi L, Morgaint B, Blanco Garcia J, Guilbaud C, Chandeysson C, et al. (2019) Bacteria from four phylogroups of the Pseudomonas syringae complex can cause bacterial canker of apricot. Plant Pathology 68(7): 1249-1258.
- Youssefi A, Hajian Shahri M (2014) Shot hole disease, survival and pathogenicity of the causal agent on stone fruit trees in Northeast Iran. Journal of Crop Protection 3(4): 563-572.
- Berge O, Monteil CL, Bartoli C, Chandeysson C, Guilbaud C, et al. (2014) A user’s guide to a data base of the diversity of Pseudomonas syringae and its application to classifying strains in this phylogenetic complex. PLoS ONE 9(9): e105547.
- Morris CE, Lamichhane JR, Nikolić I, Stanković S, Moury B (2019) The overlapping continuum of host range among strains in the Pseudomonas syringae Phytopathology Research 1(1): 4.
- Bartoli C, Berge O, Monteil CL, Guilbaud C, Balestra GM, et al. (2014) The Pseudomonas viridiflava in the Ps complex are characterized by genetic variability and phenotypic plasticity of pathogenicity-related traits. Environmental Microbiology 16(7): 2301-2315.
- Lamichhane J, Messéan A, Morris C (2015) Insights in epidemiology and control of diseases of annual plants caused by the Pseudomonas syringae species complex. Journal of General Plant Pathology 89(1): 331-350.
- Havenga M, Gatsi GM, Halleen F, Spies CF, van der Merwe R, et al. (2019) Canker and wood rot pathogens present in young apple trees and propagation material in the Western Cape of South Africa. Plant Disease 103(12): 3129-3141.
- Scortichini M (2010) Epidemiology and predisposing factors of some major bacterial diseases of stone and nut fruit trees species. Journal of Plant Pathology 92(1): 73-78.
© 2021 Iveta Pánková. This is an open access article distributed under the terms of the Creative Commons Attribution License , which permits unrestricted use, distribution, and build upon your work non-commercially.
 a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in
a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in 







.jpg)






























 Editorial Board Registrations
Editorial Board Registrations Submit your Article
Submit your Article Refer a Friend
Refer a Friend Advertise With Us
Advertise With Us
.jpg)






.jpg)














.bmp)
.jpg)
.png)
.jpg)










.jpg)






.png)

.png)



.png)






