- Submissions

Full Text
Modern Concepts & Developments in Agronomy
Important Foliar Wheat Diseases and their Management: Field Studies in Greece
Ioannis Vagelas*
Assistant Professor, Department of Agriculture Crop Production and Rural Environment, University of Thessaly, Greece
*Corresponding author: Ioannis Vagelas, Assistant Professor, Department of Agriculture Crop Production and Rural Environment, University of Thessaly, Greece
Submission: February 02, 2021Published: March 02, 2021

ISSN 2637-7659Volume8 Issue 1
Abstract
Wheat rusts and blotch diseases are notable diseases of wheat grown in temperate climates worldwide. In this research, we highlight the importance of blotch diseases on wheat in Mediterranean climates like Greece. We discuss the weather factors which influence blotch diseases of wheat development. Finally, our analyses show that applying only Propiconazole at GS37/39 wheat growth stage, Propiconazole is capable of inhibiting disease development with a significant effect on wheat yield.
Keywords: Blotch diseases; Fungicides; Wheat; Fungicide control; Leaf blotch diseases; Plant tissues
Introduction
In the last decades, many wheat varieties have developed for higher yield and disease resistance. However, about 20% of the global wheat production is lost due to diseases every year [1]. Fungal pathogens like rusts (Puccinia ssp.), Septoria leaf blotch (Septoria spp.), powdery mildew (Blumeria graminis), and Pyrenophora tritici-repentis causing tan spot are ranked among the most important wheat fungal pathogens [1]. One option, to avoid yield losses caused by these pathogens is the application of fungicides. However, the application of fungicides also depends to some extent on fungicide costs and the potentiality of applying these at the right time. This paper gives a brief explanation about wheat foliar pathogens and provides evidence that the wheat yield increases due to fungicide control of leaf blotch diseases.
Fungal diseases of wheat: Rusts
Blotch diseases: Rust pathogens and Ascomycete fungi (Family: Mycosphaerellaceae and Pleosporaceae caused the Blotch Diseases) have impeded global wheat production. Rust diseases of wheat are among the oldest and our days the epidemic losses, worldwide are rare (e.g., Brown rust in wheat is common only in Southern and Eastern parts of the UK). Wheat rusts affect significant yield losses in major producing countries such as China, India, the USA, Russia, France, Canada, Germany, Pakistan, and Australia and are not present in Greece. Septoria tritici blotch, Septoria nodorum blotch, and Tan spot are the notable diseases of wheat grown in temperate climates throughout the world, included Greece.
Wheat rusts: Three rust diseases occur in wheat; stem rust (also known as black stem rust), stripe rust (yellow), and leaf rust caused by a particular species of the genus Puccinia named P. graminis f. sp. tritici, P. striiformis f.sp. tritici and P. triticina. These diseases have similar requirements for infection (water on the leaf surface, temperatures >10 0C) and perform similar disease symptoms; yellow spots on infected plant tissues, the spots develop pustules and the pustules produce a large number of spores. When conditions are optimal for disease development, infection is achieved in 6-8 hours and uredospores - capable of causing the secondary spread of the disease - are produced in 7-10 days [2]. Early prevention methods, including cultural control practices (crop rotation), for avoiding rust diseases and the use of genetically resistant (e.g., varieties rated as “resistant”) are the traditional method for monitoring disease. In some cases, the resistant varieties show signs of susceptibility at various locations (e.g., the Middle East). However, if infection occurs on (or near) the flag leaf, fungicide treatment is required to manage i) wheat rust diseases and ii) to prevent rust epidemics. Effective fungicide groups to control wheat rusts are, Qols (Quinose outside Inhibitors-Strobilurins); DMIs (Sterol demethylation inhibitors- Azoles), and SDHIs (Succinate-dehydrogenase inhibitors). To control wheat rust disease, we use mix fungicides with different modes of action, at effective doses and correct timing [3].
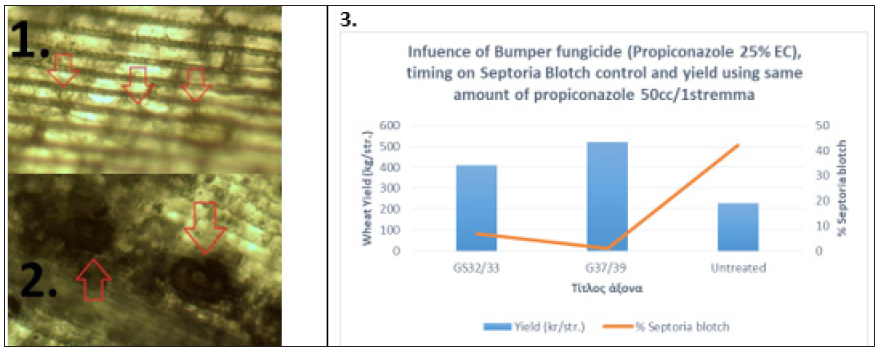
Blotch diseases (Septoria diseases and tan spot): The Ascomycete fungi pathogens, Zymoseptoria tritici (syn. Mycosphaerella graminicola or Septoria tritici), Parastagonospora nodorum (syn. Phaeosphaeria nodorum, S. nodorum, Stagonospora nodorum, or Leptosphaeria nodorum), and Pyrenophora tritici-repentis are the caused agents of wheat blotch diseases. Septoria tritici blotch (STB), is one of the most devastating diseases of wheat [4]. The fungus forms asexual fruiting bodies (pycnidia) and pycnidia appear as dark spots on the necrotic leaves (Figure 1.1) but can also be found in wheat stubble and debris where they were described to over summer on the surface of the soil [4]. Pycnidia develop underneath the stomata of infected wheat leaves (Figure 1.2 & 1.3) and remain embedded in the plant epidermis [4]. The fungus also forms sexual fruiting bodies (perithecia), which have a role in over-seasoning re-distributed by air and can spread the STB over hundreds of kilometers [4]. Depending on the environmental conditions and agricultural practice, either 2 cells asco- or macropycnidio spores can be the primary source of inoculum in STB which enters the host tissue via substomatal openings. Z. tritici does not elaborate appressoria, appear hyphae after 12-24 h in the substomatal cavity (Figure 2.1), which colonizes rapidly the mesophyll tissue of the leaf [4]. The leaves appear healthy and symptomatic plants are found 11-15 days after hyphae infection and pycnidia formation are instated in this stage (Figure 2.2). At this stage leaf cell death begins. Finally, mature pycnidia are formed, which produce the multi-cellular macropycnidiospores [4]. This type of infection, i) the spore germinates on the epidermis, ii) the fungus enters via stomata, iii) the fungus colonizes and develops biomass the leaf mesophyll, iv) symptoms appear (known as necrotrophic phase) and v) the fungus produces fruiting bodies, pycnidia were also identified for P. nodorum leaf infection (Figure 1.3). Concerning P. nodorum, germination of pycnidiospores occurred about 3h after making contact with the leaf surface and was followed by strived penetration 8-12h later [5]. Penetration was occurred through stomata and also directly through periclinal and anticlinal epidermal cell walls [5]. Once inside the leaf, the fungus extended to grow for the next 4-5 days colonizing all parts of the leaf [5]. For both pathogens, the release of ascospores and pycnidiospores was favored by rainfall. The number of air-borne ascospores increased in association with rainfall and this inoculum poses a risk to crop production and may be important to the epidemiology of Septoria diseases under climatic conditions in the wheat-producing areas [6]. Based on mycelium formation at substomatal cavities around the infection site (mentioned above), our data showed that this phase is the prevalence stage to apply DMIs (Azoles) or SDHIs fungicides. Our data (Figure 2.3), shows that at the time between GS37-39 Azoles fungicides are appropriate. We have addressed this issue from field case studies from central Greece by comparing the same amount of active ingredient Propiconazole split between pre- and post-flag leaf emergence either GS32/33+37/39 with a single application of 50cc/1stremma of Propiconazole (Bumper; Propiconazole 25% EC) at GS37/39 (Figure 2.3). Data shows that Propiconazole has a significant effect at GS37/39 on inhibiting disease development and on plant yield (Figure 2.3). Tan spot of wheat caused by the ascomycete fungus Pyrenophora triticirepentis. The fungus infects numerous perennials, grasses, and the most economically important host plants are Triticum aestivum L. and Triticum turgidum L. var. durum (durum wheat) [7]. Tan spot can induce significant yield losses by reducing the photosynthetic area, resulting in a lower thousand kernel weight and a lower number of kernels per ear [8]. P. tritici-repentis cause necroses and chloroses in wheat and this is the result of several host-specific toxins known as Ptr ToxA, Ptr ToxB, and Ptr ToxC [8]. Fungicides containing strobilurins, together with propiconazole, turned out to be very efficient in limiting the spread of tan spot [1].
Figure 1: Septoria tritici blotch leaf lesions (1.) and dark asexual fruiting bodies (1., red arrows). The presence of black asexual fruiting bodies (pycnidia) within the blotches (1., red arrows) is a diagnostic feature of Septoria tritici blotch. Pycnidia of i. Zymoseptoria tritici (2., red arrows) and ii. Parastagonospora nodorum (3., red arrows), on wheat leaf tissue, four days after incubation on moist filter paper, total objective magnification X80.
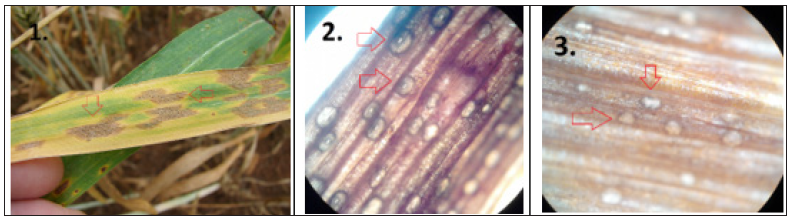
Figure 2: Mycelium formation at substomatal cavities (1., red arrows) and pycnidia development (2., red arrows) around the infection site. Influence of Propiconazole 25% EC on Septoria Blotch control (3.).
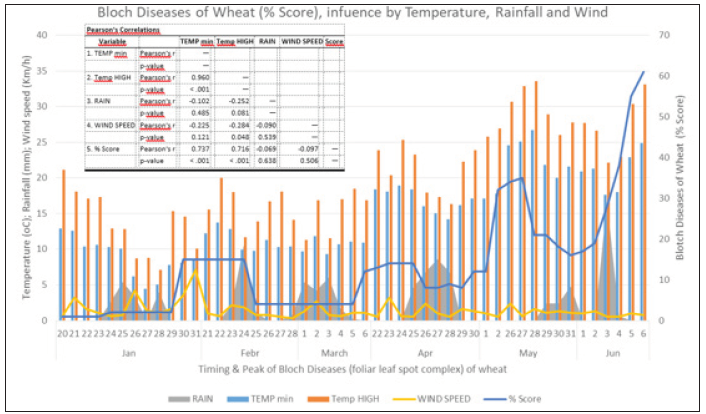
Those three important fungal leaf spot diseases of wheat, Septoria tritici blotch, Septoria nodorum blotch, and Tan spot, commonly occur in central Greece from tillering through the ripening stage of wheat (Figure 3.1 & 3.4). The fungal pathogens, Septoria tritici blotch (Figure 3.2) and Tan spot (Figure 3.3) causespecific symptoms on the leaf during the infection process. Those three pathogens (foliar leaf spot complex) can initiate infections under favorable environmental conditions e.g., temperature, rainfall, and wind (Figure 4). Symptoms develop on the leaves from tillering stage as chlorotic leaf lesions (Figure 3.1) in late January through the ripening stage (Figure 3.4) in late May. Our data show that foliar leaf spot complex influences of weather factors such as max and min temperature and by rain events. Analysis of disease progress and weather records suggested that heavy rain giving at least 10mm on 3 successive days occurred later in Jan, Apr, March, and May suggested critical conditions for the development of foliar leaf spot complex of wheat (Figure 4). Wet weather especially during April with temperatures in the range 17-24 0C is the most risk factor for greater levels of infection (Figure 4). Temperature (min, max) satisfactorily explained p< 0.001 (Pearson’s Correlations, table Figure 4) leaf blotch diseases of wheat progress at daily observations for the period 20 Jan. to 6 Jun. (Figure 4).
Figure 3: Blotch Diseases of wheat can be observed from tillering 1. Stage through ripening 2. Septoria tritici blotch (2., red arrow) occurring with Tan spot (3., red arrow). 3. During ripening stage blotch diseases lesions of wheat, on higher leaves usually follow veins 4. With straight edges and a clear yellow margin.

Figure 4: Daily mean values of temperature (0C), rainfall (mm), wind speed (km/h), and blotch diseases of wheat (% score, blue line) in central Greece.
Conclusion
Overall, we can conclude that control Septoria Blotch had a significant effect on wheat yield. Propiconazole application at GS 37‐39 stages of wheat satisfactorily explained yield increase by controlling the disease. Mean temperature and rainfall from the tillering through the ripening stage are important factors for foliar leaf spot complex diseases of wheat.
References
- Serfling A, Kopahnke D, Habekuss A, Novakazi F, Ordon F (2017) Wheat diseases: an overview. Achieving sustainable cultivation of wheat, (1st edn).
- https://aces.nmsu.edu/pubs/_a/A415/welcome.html
- https://ahdb.org.uk/knowledge-library/fungicide-resistance-management-in-cereals
- Steinberg G (2015) Cell biology of Zymoseptoria tritici: Pathogen cell organization and wheat infection. Fungal Genet Biol 79: 17-23.
- Solomon PS, Wilson TG, Rybak K, Parker K, Lowe RG, et al. (2006) Structural characterisation of the interaction between Triticum aestivum and the dothideomycete pathogen Stagonospora nodorum. European Journal of Plant Pathology 114(3): 275-282.
- Cordo CA, Simon MR, Perelló AE, Alippi HE (1999) Spore dispersal of leaf blotch pathogens of wheat (Mycosphaerella graminicola and Septoria tritici). Septoria and Stagonospora Diseases of Cereals, pp: 98-101.
- Strelkov SE, Lamari L (2003) Host-parasite interactions in tan spot [Pyrenophora tritici-repentis] of wheat. Canadian Journal of Plant Pathology 25(4): 339-349.
- Faris JD, Liu Z, Xu SS (2013) Genetics of tan spot resistance in wheat. Theoretical and Applied Genetics 126(9): 2197-2217.
© 2021 Ioannis Vagelas. This is an open access article distributed under the terms of the Creative Commons Attribution License , which permits unrestricted use, distribution, and build upon your work non-commercially.
 a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in
a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in 







.jpg)






























 Editorial Board Registrations
Editorial Board Registrations Submit your Article
Submit your Article Refer a Friend
Refer a Friend Advertise With Us
Advertise With Us
.jpg)






.jpg)














.bmp)
.jpg)
.png)
.jpg)










.jpg)






.png)

.png)



.png)






