- Submissions

Full Text
Modern Concepts & Developments in Agronomy
Effect of Time of Harvesting and Disease Resistance in Reducing Cassava (Manihot esculenta Crantz) Yield Losses by Two Viral Diseases
Midatharahally N Maruthi1*, Bernadetta Kimata2, Emily A Masinde3 and Geoffrey Mkamilo4
1Natural Resources Institute, University of Greenwich, Chatham Maritime, Kent ME4 4TB, UK
2Tanzania Agricultural Research Institute (TARI)-Naliendele, P.O Box 509, Mtwara, Tanzania
3Kenya School of Agriculture, P.O. Box 1909-10100, Nyeri, Kenya
4Tanzania Agricultural Research Institute (TARI)-Makutupora, P.O Box 1571, Dodoma, Tanzania
*Corresponding author: Midatharahally N Maruthi, Natural Resources Institute, University of Greenwich, Chatham Maritime, Kent ME4 4TB, UK
Submission: February 13, 2020Published: March 10, 2020

ISSN 2637-7659Volume 6 Issue 1
Abstract
Cassava mosaic disease (CMD) and cassava brown streak disease (CBSD) are two important biotic constraints for cassava (Manihot esculenta Crantz) production in Eastern and Southern Africa. CMD causes a general decline in yield in affected plants of susceptible cassava varieties but CBSD causes rotting of edible roots. Delayed harvesting can increase rotting of roots and making them unfit for consumption or marketing, and thus affecting the livelihoods of poor farmers. This study investigated the effect of interaction between time of harvesting and levels of disease resistance to identify ideal harvesting times for reducing yield losses. The resistant cassava variety Namikonga remained in the field for the duration of the study, up to 24 months after planting without incurring significant yield losses, while the tolerant varieties Kiroba and Kizimbani could only be maintained up to 21 months. Susceptible varieties Mreteta and Albert suffered significant yield losses beyond 15 months. Among the varieties, Kizimbani had the least CBSD and CMD foliar symptoms as well as farmer desirable traits including high root weight, quantity of marketable roots and dry matter content. Harvesting of cassava can depend on the resistance or susceptibility of the varieties grown. Therefore, the above harvesting times for different varieties were recommended for minimizing yield losses due to the diseases and thus maximizing yields to the farmers.
Abbreviations: CBSD: Cassava Brown Streak Disease; CBSIs: Cassava Brown Streak Ipomoviruses; CBSV: Cassava Brown Streak Virus; CMBs: Cassava Mosaic Begomoviruses; CMD: Cassava Mosaic Disease; CMV: Cassava Mosaic Virus; UCBSV: Uganda Cassava Brown Streak Virus
Introduction
Cassava (Manihot esculenta Crantz) roots are an important staple food in sub-Saharan Africa (SSA) providing daily source of carbohydrates for over 450 million [1,2]. Apart from utilization as fresh roots, it can also be processed into flour, which may be consumed by the farmers, sold in market, or can be used in bakery, starch or ethanol production and paper making [3]. Cassava tolerates unpredictable drought periods and can grow on marginal soils with minimum inputs. Subsistence farmers rely on cassava as a vital source of energy since it can be harvested throughout the year. In addition, the average cassava yield in East and Southern Africa has, however, remained low since the 1990s, rarely exceeding 10.0t/ha, which is far below the estimated yield potential of 50-60t/ha [4,5]. Among the numerous factors, the biotic stresses: cassava mosaic disease (CMD) and cassava brown streak disease (CBSD) have greatly contributed to low cassava productivity in SSA [6-8].
CBSD is caused by two RNA viruses belonging to the genus Ipomovirus in the family Potyviridae: cassava brown streak virus (CBSV) and Ugandan cassava brown streak virus (UCBSV) [7,9-11], which are together called cassava brown streak ipomoviruses (CBSIs) [12]. CBSD symptoms are characterized by leaf chlorosis along the secondary and tertiary veins, and elongated necrotic lesions on stems [8,13,14]. The major economic damage arises from the dry, . In southern coastal Tanzania, for example, yield losses of up to 70% were caused by CBSD in susceptible cultivars [15]. The CMD is caused by 11 cassava mosaic begomoviruses (CMBs), (Family Geminiviridae: Genus Begomovirus) [7,16].
CMD symptoms typically include irregular yellow or yellowgreen chlorotic mosaic pattern on leaves, leaf distortion, stunted plant growth and reduced root yields, but not rotting of roots [17- 19]. Losses up to 100% have been reported in highly susceptible varieties [19,20] or in mixed infections of CMD and CBSD [21,22]. CMD-resistant varieties have been developed which express less severe symptoms than susceptible ones especially during the late stages of plant growth, when the resistant varieties become virtually symptomless [23]. Apart from plant genotype, environmental factors also influence symptom expression and leaves produced during periods of cool weather tend to have more severe symptoms than those produced under hotter conditions [24]. Moreover, some strains of viruses cause more severe symptoms than others and have greater effects on growth and yield [25].
Dual infections of CMD and CBSD are common and they are a serious threat to cassava production and food security in SSA. Deployment of cassava varieties with dual resistance to both diseases is the only sustainable way to control [26]. More recently, breeding has been focussing on varieties with dual resistance to both CMD and CBSD. Crossing the resistant cassava variety (var.) Namikonga (CBSD resistant but CMD susceptible) with var. AR42- 4 (CBSD susceptible but CMD resistant) developed a new cassava hybrid Pwani which is resistant to CMD but tolerant to CBSD with no or delayed root necrosis [27]. However, the large-scale adaption of such varieties is yet to be achieved in the worst affected countries of eastern and southern Africa [7,16]. A property unique to cassava is its ability to store the roots in the ground beyond the optimum harvesting time without major reduction in yields. Cassava roots can stay underground for up to three years after planting, which is making it an important food security crop available at the time of needs when all other crops are not available [28]. Many farmers value long ‘in-ground storage’ of cassava roots especially in drought prone areas as they can harvest a few plants at a time (piecemeal harvesting) leaving others “stored” in the field for later harvest [29]. Preferences for long in-ground storage of cassava roots have been reported in Tanzania, Uganda, and Malawi [30,31]. Additionally, to ensure food security during adverse conditions, cassava farmers in Tanzania plant both early maturing (6-9 months) and late maturing (12-18 months) varieties [31]. However, all the food security benefits of cassava are lost due to the infections of CBSD as root necrosis becomes increasingly severe with plant age and late harvesting which can result in significant yield losses.
In many countries, farmers have adopted early harvesting to cope with such CBSD losses and thus reducing the food security value of cassava [15,30,32,33]. Early harvesting, especially for varieties that accumulate starch late (late bulking) contributes to significant yield losses [33]. Cassava is harvested normally between 9 and 12 months after planting but little information is available on the optimum time of harvesting to reduce the impact of CBSD. To identify optimum harvesting times for cassava varieties with different levels of resistance to the two diseases, this study was carried out with the following objectives;
(i) Determine the effect of harvesting time on cassava yields (root weight, marketable roots and dry matter content) in a 24 months growth cycle, and
(ii) Testing the resilience of CBSD -resistant, -tolerant and -susceptible varieties to root necrosis damage and yield losses.
These results will help develop recommendations for farmers on the ideal harvesting times for different cassava varieties in disease affected areas.
Material and Methods
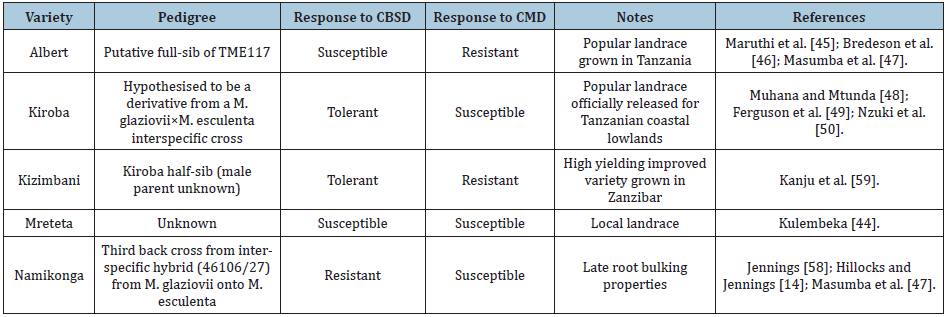
Five popular cassava varieties; Namikonga, Kiroba, Kizimbani, Albert and Mreteta, grown in different regions of Tanzania were selected for evaluation based on their reaction to CMD and CBSD (Table 1). The field trial was established in February 2014 in the CMD and CBSD hot spot research fields of the Tanzania Agricultural Research Institute (TARI)-Naliendele in the Mtwara region of southern Tanzania. TARI-Naliendele lies on the coastal belt of the Indian Ocean and is located at 10° 22′ 20“S, 40° 10′ 34“E and 111m above mean sea level. The area receives the main rainfall from December-May with second rains of scattered showers in August-October (TMA, 2009). The sandy soils of Mtwara region are considered poor for most crops. They are characterised by deep, well drained, weak structured, dark reddish-brown loamy sand topsoil over reddish brown moderately structured sandy loam to sandy clay loam subsoil [34].
Table 1: Pedigree of Tanzanian cassava varieties used to estimate the effect of CMD and CBSD, and the time of harvesting on cassava yield losses.
Experimental design
A split plot design with three replicates was used for the study. The varieties in the main plots were randomly assigned in replications while the different harvesting times were sub-plots randomized within the main plots. The main plots each measured 80m long and 16m wide while the sub-plots each measured 10m long and 2m wide. Mature cassava cuttings of about 25cm long and having 4-5 nodes with viable buds were collected for each variety from TARI-Makutupora in Tanzania (a disease-free site used for seed multiplication). To increase disease inoculum, CMD susceptible var. Limbanga and CBSD susceptible var. Albert were planted around the experimental plots as spreader rows [35]. The trial was rain fed, kept weed free by monthly weeding and no fertilizer or chemical pesticides were applied.
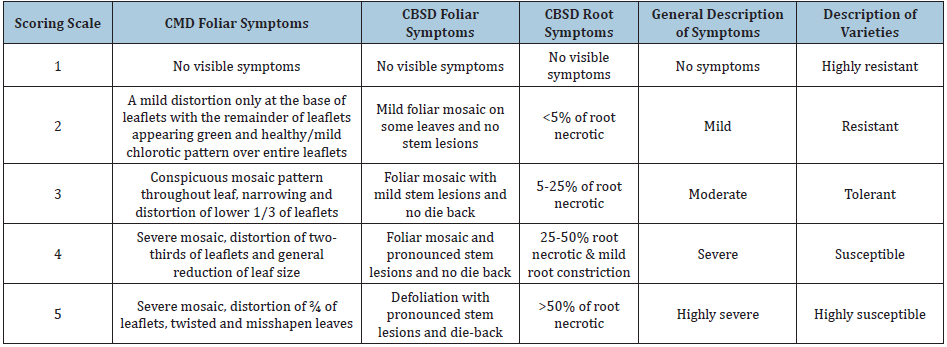
Estimating the impact of CMD and CBSD on cassava yieldsData on several parameters required to assess the impact of CMD and CBSD on cassava yields were collected at 6, 9, 12, 15, 18, 21, and 24 months after planting. These included CMD and CBSD foliar incidences, foliar severities, root necrosis, yield loss, root weight (t/ha), marketable roots (t/ha) and dry matter content of cassava roots. Foliar incidences were calculated as a percentage of the plants showing symptoms while foliar severities were recorded based on a scale of 1-5 for both CMD and CBSD according to Hahn et al. [36] and Hillocks et al. [29], respectively (Table 2). Roots from each plant were harvested and chopped longitudinally and transversely to check for root necrosis on the starch bearing tissues. Scoring for root necrosis was done based on a 1- 5 scale by Gondwe et al. [30]. Root weight in tonnes per hectare (t/ha) was estimated according to Kamau et al. [37].
Table 2: CMD and CBSD foliar severity scoring scale.
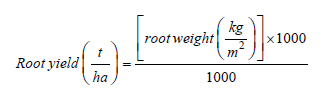
All roots with necrosis score of ≤2 was considered marketable as only tiny spots of root necrosis were observable at this score [38]. Severe root necrosis affects root quality, therefore reducing the quantity of marketable roots (t/ha). Marketable roots were determined by deducting the unmarketable roots which had root necrosis score >2. Each category was weighed separately, and yield loss was calculated by expressing the weight of the unmarketable roots (t/ha) as a percentage of the total root weight (t/ha). Further, data were collected on root dry matter content using the specific gravity method [39].
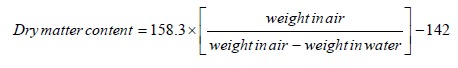
All data were subjected to ANOVA to obtain the contribution of all sources of variation to the total sum of squares. The analysis was carried out using the PROC GLM procedure of SAS 13.1 [40] (SAS Institute Inc, 2013) and means for varieties and harvest times were separated using Tukey’s HSD test at 95% confidence level. Graphs were also plotted to show the trend of disease incidences, severities, and yield losses for different varieties.
Result
CMD foliar incidence varied significantly (P≤0.05) among the varieties (Figure 1). Mreteta maintained the highest incidence ranging from 47.5 to 90.0%, while Kizimbani and Albert had the least, rarely exceeding 10.0% throughout the growing season. Kiroba and Namikonga had moderate incidence ranging from 19.7 to 52.9%. Similarly, CMD foliar severity varied significantly (P≤0.05) among the varieties (Figure 1). Mreteta and Kiroba had the highest severity ranging from 2.0 to 3.5 while Kizimbani and Albert had the least ranging from 1.0 to 1.1 throughout the growing season. Moderate severity was recorded on Namikonga. Combined means for all time points showed a similar trend where both CMD foliar incidence and severity increased from 6 MAP, peaked at 15 MAP then dropped gradually till the end of the growing season at 24 MAP (Table 3).
Figure 1: CMD/CBSD incidence and severity of cassava varieties at different harvesting times from 6-24 months after planting (MAP). Vertical bars denote the standard errors of the means with 95% confidence interval.
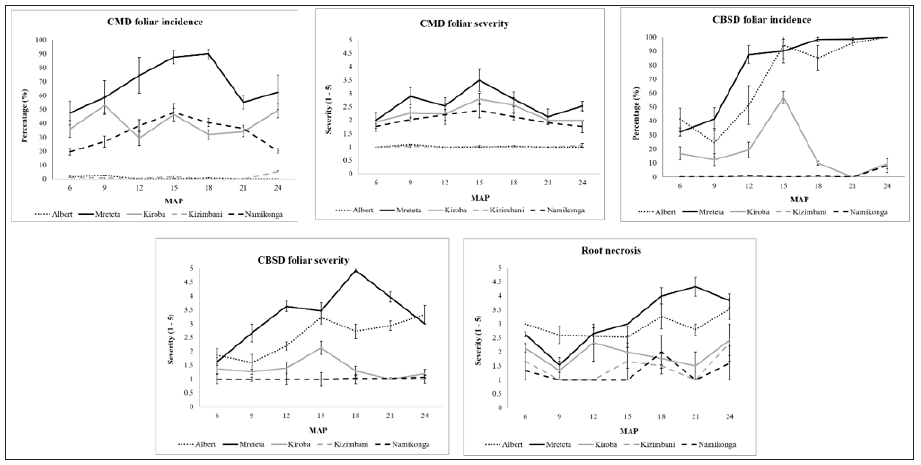
Table 3: Combined means of disease and yield traits for different times of harvesting (6 to 24 months after planting).
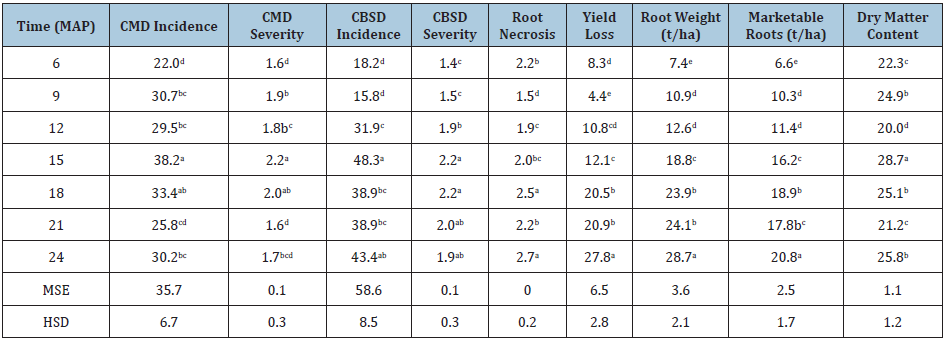
MSE=mean square error, HSD: tukey’s honest significant difference; different letters indicate that means within a column are significantly different (P≤0.05).
CBSD foliar incidences and severityCBSD foliar incidence also varied significantly (P≤0.05) among the cassava varieties. The highest incidences were recorded on Albert and Mreteta, which gradually increased throughout the 24 months growing period and ranged from 25.0 to 100% (Figure 1). Namikonga and Kizimbani maintained low foliar CBSD incidences not exceeding 10.0% while Kiroba had intermediate incidence ranging from 9.5 to 57.1%. Similarly, CBSD foliar severity increased throughout the growing period on Albert and Mreteta with the highest severity ranging from 1.6 to 4.9 while Namikonga and Kizimbani maintained the least <1.5 (Figure 1). Kiroba had an intermediate foliar severity ranging from 1.3 to 2.1. Comparable to CMD foliar incidence and severity, combined means for all time points showed a similar trend for CBSD foliar incidence and severity, which increased from 6 MAP, peaked at 15 MAP then decreased gradually till 24 MAP (Table 3).
Root damage by CBSD infectionCBSD root necrosis and rotting varied significantly (P≤0.05) among the varieties (Figure 1). Mreteta and Albert had the most damaged roots with severities ranging from 1.5 to 4.3 throughout the growing season. Namikonga had the least damage with severity not exceeding 1.7 while Kizimbani and Kiroba had intermediate severities ranging from 1.0 to 2.3. Combined means for all the harvesting time points showed that root necrosis was high at 6 MAP with a severity of 2.2. The severity decreased to 1.5 at 9 MAP before beginning to rise again from 1.9 at 12 MAP to 2.7 at 24 MAP (Table 3).
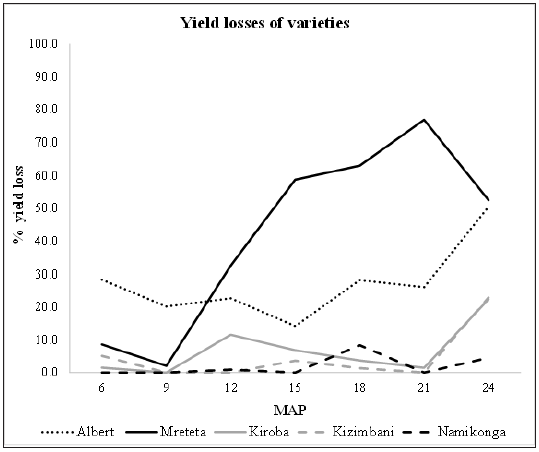
Yield losses due to CBSD infectionYield losses occur due to necrosis and rotting of infected roots and in this study, losses varied among the varieties (Figure 2). Mreteta had the highest losses with a maximum of 76.9% at 21 MAP. Although Albert had moderate root losses of <30.0% between 6-21 MAP, it nevertheless had a high loss of 50.4% at 24 MAP. Kizimbani and Kiroba maintained low losses of <12.0% between 6-21 MAP, but the losses increased to 23.0% at 24 MAP. Finally, Namikonga had the least losses of <10.0% throughout the growing season. Combined means for all the harvesting time points showed that losses reduced at 9 MAP before rising again at 12 MAP till the end of the growing season at 24 MAP (Table 3).
Figure 2: Means for yield losses of varieties at different harvesting times from 6 to 24 months after planting (MAP).
Measurement of cassava yield traits
The root weight increased throughout the growing season and the cumulative root weight varied significantly for each variety (P≤0.05) (Tables 4&5). Kizimbani and Kiroba had significantly highest cumulative root weight of 21.0t/ha and 20.8t/ha, respectively, while Namikonga had the least at 14.7t/ha (Table 4). The mean marketable roots increased throughout the growing season and cumulative marketable roots for each variety varied significantly (P≤0.05) (Tables 4&6). Kizimbani had the highest cumulative marketable roots of 19.7t/ha and was significantly different from Kiroba (15.9t/ha), Namikonga (14.3t/ha), Albert (12.0t/ha) and Mreteta (10.9t/ha). The mean dry matter content fluctuated throughout the growing season depending on the weather patterns (Table 7) (Figure 3). Low dry matter content was recorded in months with high rainfall for example at 12 and 21 MAP and it increased in the rest of the drier months including at 9, 15 and 24 MAP. The highest cumulative dry matter content was recorded in Namikonga at 24.7% and was not significantly different from Kiroba (24.4%) and Kizimbani (24.3%) (Table 3). Albert (23.7%) and Mreteta (22.7%) had the lowest dry matter content and were not significantly different from each other.
Figure 3: Variability in rainfall and temperature for 2014/2015 and 2015/2016 growing season.
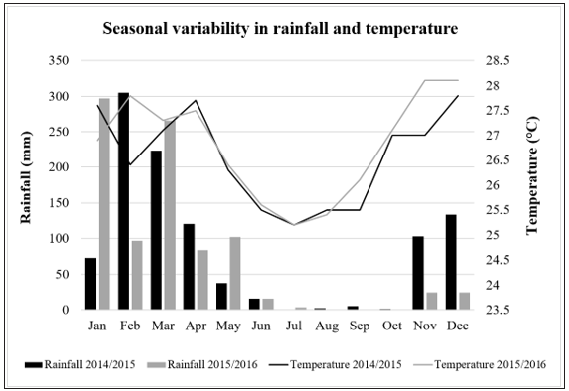
Table 4: Combined means of disease and yield traits for cassava varieties.
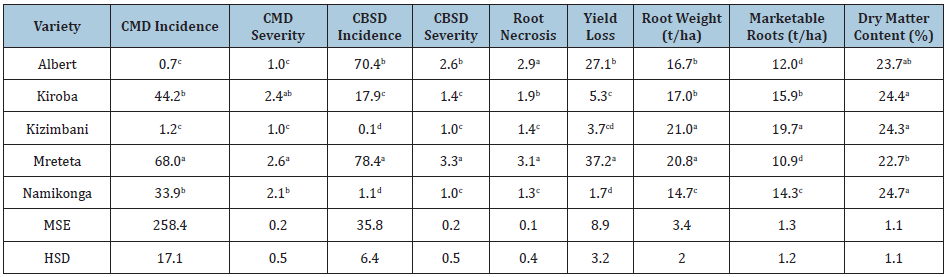
MSE=mean square error, HSD: tukey’s honest significant difference; different letters indicate that means within a column are significantly different (P≤0.05).
Table 5: Mean root weight (t/ha) and standard errors of means (SEM) for varieties at different harvesting times from 6 to 24 months after planting (MAP).
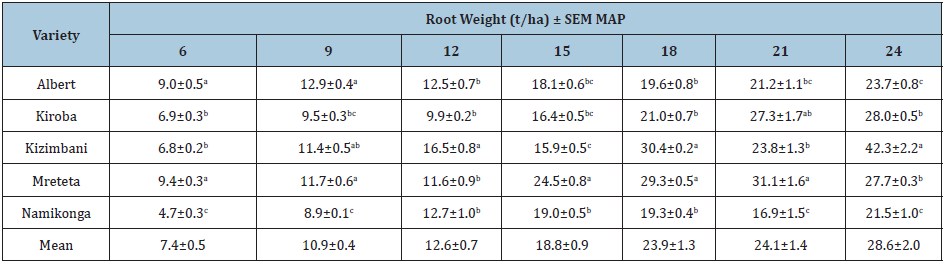
±=95% confidence interval for means; different letters indicate that means within a column are significantly different (P≤0.05).
Table 6: Mean marketable roots and standard errors of means (SEM) for varieties at different harvesting times from 6 to 24 months after planting (MAP).

±=95% confidence interval for means; different letters indicate that means within a column are significantly different (P≤0.05).
Table 7: Mean % dry matter content and standard errors of means (SEM) for cassava varieties at different harvesting times from 6 to 24 months after planting (MAP).
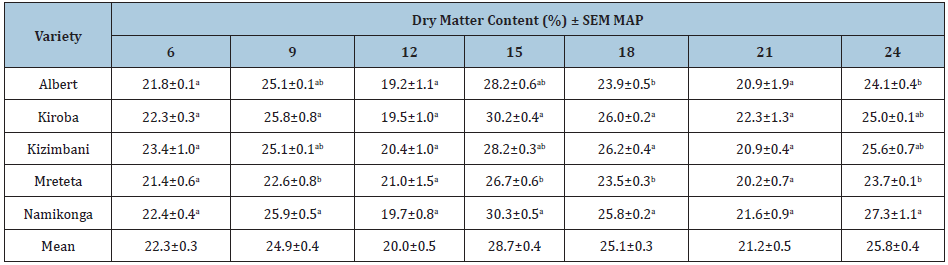
±=95% confidence interval for means; different letters indicate that means within a column are significantly different (P≤0.05).
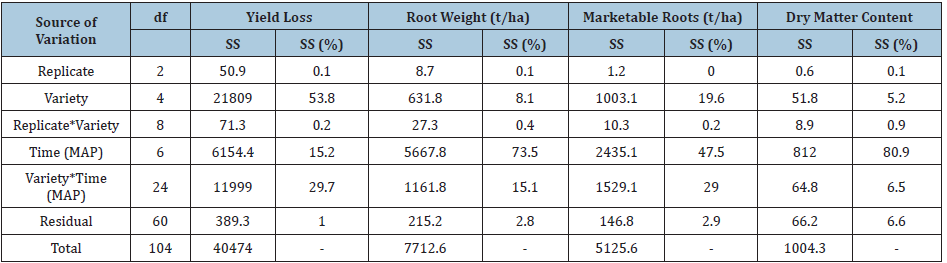
Sum of squares (SS) for traits evaluated
The ANOVA revealed that a larger percentage of total sum of squares (SS) ranging from 53.8 to 81.9% was attributed to variety for foliar incidence and severity for both diseases, root necrosis and yield loss (Tables 8&9). The larger SS indicated that the genetic makeup of the varieties highly influenced the expression of disease symptoms. On the contrary, a larger percentage of SS ranging from 47.5 to 80.9% was attributed to time for yield traits evaluated including: root weight, marketable roots, and dry matter content. This indicated that time contributed to the most variations observed in the yield traits analysed. Lastly, variety and time interaction had the least SS (6.5-29.7%) for all traits analysed since it had minimal effect on trait expression.
Table 8: Sum of squares for CMD and CBSD foliar incidence, severity and root necrosis.
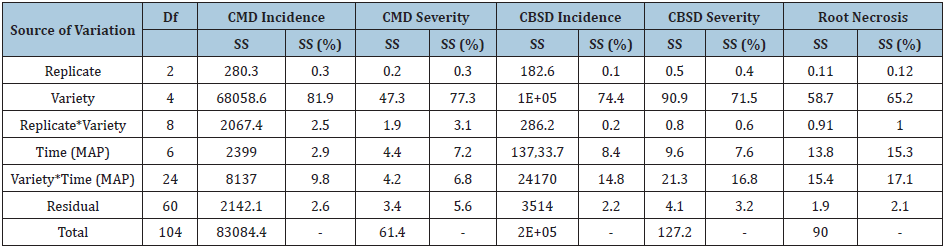
Table 9: Sum of squares for yield loss, root weight, marketable roots, and dry matter content.
Discussion
Cassava production in eastern and southern Africa is severely constrained by two major viral diseases: CMD and CBSD. Deployment of varieties resistant to both diseases is urgent and is the only sustainable way to control the diseases. Cassava is an important food security crop in Africa due to its ability to remain in the ground without deterioration for more than two years after reaching physiological maturity [28]. Farmers keep cassava in the ground typically for 12 to 18 months and practice piece meal harvesting for home consumption when all other crops not available [30,31]. However, the contribution of cassava to food security has been under severe threat by CBSD because it causes rotting of affected roots in susceptible varieties. To make matters worse, the damage by CBSD will not be apparent until the crop is harvested, and roots processed for cooking. This can be a sudden loss of the only source of food for millions of households relying solely on cassava during extreme times such as droughts. Delayed harvesting increases CBSD severity and the number of affected roots, thus farmers can no longer depend on cassava for food. To minimize CBSD damage, farmers have adopted early harvesting before the full physiological maturity of cassava plants. Although this may reduce CBSD damage, however, it can significantly reduce yields due to the low accumulation of starch in root tissues. Understanding the effect of time of harvesting and the levels of resistance of cassava varieties to both CMD and CBSD is therefore important for safeguarding the food security of millions of poor farmers. In this study, we investigated the interaction between times of harvest with resistance levels of the cassava varieties for identifying optimum cassava harvest times. The genetic make-up of the cassava varieties had the highest SS, therefore contributing significantly to the variations observed in disease traits including incidences and severity of foliar and root symptoms for both diseases. This is good news because the findings indicated that the traits are heritable and thus can be selected in breeding programmes. The foliar disease incidences and severities increased till 15 MAP, during active plant growth, and then declined till 24 MAP. This confirms that CMD symptoms decrease with increasing plant age as the older leaves are less susceptible than new growth [41]. Similarly, CBSD foliar symptoms mask in older plants and are difficult to recognize as the lower leaves with prominent symptoms senesce and fall off [42].
On the contrary, root necrosis severity and yield losses increased with delayed harvesting as expected. Chipeta et al. [43] reported similar findings where yield loss due to CBSD was significantly associated with increased root severity at different harvest times and yield loss increased from 10.9% to 43.1% between 6 and 12 MAP. Var. Mreteta was the most affected with the maximum foliar incidences of >90.0% for both CMD and CBSD. It also had the highest foliar and root necrosis severities ranging from 3.5 to 4.9, confirming that Mreteta is susceptible to both diseases [44]. Albert showed low and high foliar incidences and severity for CMD and CBSD, respectively, which confirms its classification as CMD-resistant but CBSD-susceptible [45-47]. Although susceptible to CMD, Kiroba has been previously reported to be tolerant to CBSD since it expresses CBSD foliar symptoms with delayed or no root symptoms [48-50]. Kizimbani showed minimal CMD and CBSD foliar incidences (<6.0%) and severities (<1.1) but with moderate root necrosis severity (<2.3). Namikonga had the lowest CSBD foliar incidence (<8.2%), severity (<1.1) together with low root necrosis severity (<1.6).
However, it had moderate CMD foliar severity and incidences of up to 51.7%, indicating it’s susceptibility to CMD. Namikonga has perpetually exhibited no or low CBSD symptoms severity for many years and has been used as one of the best CBSD progenitors in breeding programmes [45,47,51-53]. High root necrosis severity resulted in high yield losses at different time points [54]. The average yield loss was 8.3% at 6 MAP, which dropped to 4.4% at 9 MAP, then began rising again up to a maximum of 27.8% at the end of the experiment. Development of the fibrous roots of cassava into starchy tubers occurs between 2 and 9 MAP depending on the varieties [54]. Thereafter, tuberization stops and root bulking begins where the tubers increase in size and weight. At 6 MAP it is too early for harvesting most cassava varieties and thus the roots were fewer and smaller in size. CBSD infection at this stage can result in significant loss of the root in susceptible varieties and thus the losses can be proportionately higher. The yield losses decreased at 9 MAP mainly because this was the active bulking period for cassava and thus the tubers can outgrow the damage caused by the disease. Farmers actively remove the minimally damaged portions of such roots and use the remaining healthy part for consumption which is contributing to the lower losses observed at 9 MAP. Moreover, during this period of active growth, several tubers escape disease infection and develop into completely healthy tubers, further contributing to observed lower losses. Time of harvest and disease resistance affected yield losses due to CBSD in different varieties [55]. The susceptible var. Mreteta developed the most severe root necrosis throughout the growing season and had the highest yield losses with a maximum of 80.0% at 21 MAP. On the contrary, the resistant var. Namikonga was least affected with correspondingly low losses not exceeding 9.0%. Tolerant var. Kiroba and Kizimbani developed moderate root necrosis therefore maintaining low losses below 12.0% between 6 and 21 MAP. The losses, however, increased to approximately 23.0% at 24 MAP in both varieties. Comparable to the tolerant varieties, the CBSD-susceptible var. Albert maintained yield losses below 30.0% from 6 to 21 MAP but 50.0% of its yield was lost during harvesting at 24 MAP. By 24 MAP, root bulking has stopped, and rapid virus accumulation may occur in the roots causing losses in tolerant and susceptible varieties. Root necrosis severity and yield loss were fluctuating in Kiroba, Kizimbani and Namikonga indicating the recovery of resistant and tolerant varieties to virus infection or localised infection in any given plant tissue [45].
Time of harvest highly influenced root weight as it increased throughout the growing season. The maximum root weights for most of the varieties were recorded at 24 MAP and they included Kizimbani (42.3t/ha), Mreteta (31.1 t/ha), Kiroba (28.0t/ha), Albert (23.7t/ha) and Namikonga (21.5t/ha). Similarly, the quantity of marketable roots increased with delayed harvesting apart from the times when the varieties had severe root necrosis and high yield losses. High root weights coupled with low yield losses resulted in high quantities of marketable roots [54]. The highest quantity of marketable roots was recorded in Kizimbani and Kiroba which are high yielding CBSD-tolerant varieties. Although Namikonga had the least losses, its marketable root quantity was significantly lower than that of Kizimbani and Kiroba since it is a late bulking and low yielding variety [56]. Albert was severely affected by CBSD; therefore, it had low quantities of marketable roots. Mreteta had the highest root weights but had the least marketable roots due to high susceptibility to CBSD. Although Mreteta was severely affected by both CBSD and CMD it still maintained a higher root weight not significantly different from Kizimbani. In the absence of diseases, Mreteta has the potential of producing higher yield. These findings emphasized on the importance of deploying varieties that are both disease resistant and high yielding to increase the quantity of marketable roots.
Dry matter content varied depending on the time of harvest. High mean dry matter content was recorded during low rainfall including at 9 MAP (24.9%), 15 MAP (28.7%), 18 MAP (25.1%) and 24 MAP (25.8%). On the contrary, low mean dry matter content was recorded at 12 MAP (20.0%) and 21 MAP (21.2%), which are periods characterised by high rainfall. During the rainy season, cassava roots absorb more water which results in proportionally low dry matter content (Masinde et al., 2017). Additionally, varieties with the least CBSD root symptoms had the highest dry matter content (eg. Namikonga, Kiroba and Kizimbani) and vice versa (eg. Albert and Mreteta). This indicates that the presence of CBSD symptoms on either leaves or roots affects key agronomic traits such as dry matter content leading to loss in farmer desirable traits [54]. A combination of timely harvesting and deployment of resistant/ tolerant varieties can reduce cassava losses due to CMD and CBSD. The recommended time of harvest for officially released varieties is 12 MAP although farmers prefer long ‘in-ground storage’ of cassava roots especially in drought prone areas. This study showed that beyond 12 MAP, CBSD resistant var. Namikonga could be harvested till 24 MAP because it had the least CBSD root losses throughout the growing season [57-60]. Tolerant vars. Kizimbani and Kiroba could stay in the field up to 21 MAP without significant losses while susceptible vars. Albert and Mreteta would be best harvested at or before 15 MAP. Although we do not recommend growing susceptible varieties, this study has also demonstrated that satisfactory yields can still be obtained from all varieties when using disease-free cassava as planting material and harvested no later than 15 MAP. The recommended harvesting time points maximize both root weight and marketable roots for different varieties categorised as resistant, tolerant, and susceptible. Therefore, each variety can be harvested at its own appropriate time depending on its resilience to CBSD.
Conclusion
The findings in this study have shown that CBSD -resistant, -tolerant, and -susceptible varieties can be harvested at different times to minimise CBSD root necrosis damage and yield losses. When disease-free planting materials are used for cultivation, the resistant, tolerant and susceptible cassava varieties can be left in the ground for up to 24, 21 and 15 months, respectively, without incurring significant losses to cassava yields. These results help seed specialists, agricultural extension officers to provide specific recommendations to farmers on each cassava variety grown, and thus maximising the food security value of cassava in the worst affected regions of eastern and southern Africa.
Acknowledgement
This work was funded as part of the projects AURG/2/141 and AURG II-1-060-2016, both funded by the African Union Commission to the University of Greenwich.
References
- Nassar NMA, Ortiz R (2017) Cassava improvement: challenges and impacts. J Agric Sci 145(2): 163-171.
- Jarvis A, Villegas JR, Campo BVH, Racines CN (2012) Is cassava the answer to African climate change adaptation? Trop Plant Biol 5: 9-29.
- Food and Agriculture Organisation (2013) Save and grow cassava: Harvest, post-harvest and value addition. A guide to sustainable production intensification, FAO, Rome, Italy.
- Fermont AM, Van Asten PJ, Tittonell P, Van Wijk MT, Giller KE (2019) Closing the cassava yield gap: an analysis from smallholder farms in East Africa. Field Crops Res 112(1): 24-36.
- FAOSTAT (2017) Crops 2018: Production statistics-crops. FAO, Rome, Italy.
- Bull SE, Ndunguru J, Gruissem W, Beeching JR, Vanderschuren H (2011) Cassava: constraints to production and the transfer of biotechnology to African laboratories. Plant Cell Rep 30(5): 779-787.
- Legg JP, Jeremiah SC, Obiero HM, Maruthi MN, Ndyetabula I, et al. (2011) Comparing the regional epidemiology of the cassava mosaic and cassava brown streak virus pandemics in Africa. Virus Res 159(2): 161-170.
- Tomlinson KR, Bailey AM, Alicai T, Seal S, Foster GD (2018) Cassava brown streak disease: historical timeline, current knowledge and future prospects. Mol Plant Pathol 19(5): 1282-1294.
- Winter S, Koerbler M, Stein B, Pietruszka A, Paape M, et al. (2010) Analysis of cassava brown streak viruses reveals the presence of distinct virus species causing cassava brown streak disease in East Africa. J Gen Virol 91(Pt 5): 1365-1372.
- Vanderschuren H, Moreno I, Anjanappa RB, Zainuddin IM, Gruissem W, et al. (2012) Exploiting the combination of natural and genetically engineered resistance to cassava mosaic and cassava brown streak viruses impacting cassava production in Africa. PLoS One 7(9): e0045277.
- Ndunguru J, Sseruwagi P, Tairo F, Stomeo F, Maina S, et al. (2015) Analyses of twelve new whole genome sequences of cassava brown streak viruses and Ugandan cassava brown streak viruses from East Africa: diversity, supercomputing and evidence for further speciation. PloS One 10: e0139321.
- Maruthi MN, Jeremiah SC, Mohammed IU, Legg JP (2017) The role of the whitefly, Bemisia tabaci (Gennadius), and farmer practices in the spread of cassava brown streak ipomoviruses. J Phytopathol 165(11-12): 707-717.
- Nichols RFJ (1950) The brown streak disease of cassava: distribution climatic effects and diagnostics symptoms. E Afr Agric For J 15(3): 154-160.
- Hillocks RJ, Jennings DL (2003) Cassava brown streak disease: a review of present knowledge and research needs. Int J Pest Manage 49(3): 225-234.
- Hillocks RJ, Raya MD, Mtunda K, Kiozia H (2001) Effects of brown streak virus disease on yield and quality of cassava in Tanzania. J Phytopathol 149(7-8): 389-394.
- Legg JP, Kumar PL, Makeshkumar T, Tripathi L, Ferguson M, et al. (2015) Cassava virus diseases: biology, epidemiology, and management. Adv Virus Res 91: 85-142.
- Storey HH, Nichols RFW (1938) Studies of the mosaic diseases of cassava. Ann Appl Biol 25(4): 790-806.
- Thresh JM, Cooter RJ (2005) Strategies for controlling cassava mosaic virus disease in Africa. Plant Pathol 54(5): 587-614.
- Tembo M, Mataa M, Legg J, Chikoti PC, Ntawuruhunga P (2017) Cassava mosaic disease: incidence and yield performance of cassava cultivars in Zambia. J Plant Pathol 99(3): 681-689.
- Thresh JM, Fargette D, Nape GWO (1994) The viruses and virus diseases of cassava in Africa. Afr Crop Sci J 2(4): 459-478.
- Fondong VN, Pita JS, Rey ME, de Kochko A, Beachy RN, et al. (2000) Evidence of synergism between African cassava mosaic virus and a new double-recombinant geminivirus infecting cassava in Cameroon. J Gen Virol 81(1): 287-297.
- Pita JS, Fondong VN, Sangaré A, Nape GWO, Ogwal S, et al. (2001) Recombination, pseudo-recombination and synergism of geminiviruses are determinant keys to the epidemic of severe cassava mosaic disease in Uganda. J Gen Virol 82(Pt 3): 655-665.
- Jennings DL (1957) Further studies in breeding cassava for virus resistance. E Afr Agric For J 22(4): 213-219.
- Gibson RW (1994) Long‐term absence of symptoms in heat‐treated African cassava mosaic. Trop Sci 34(1): 154-158.
- Owor B, Legg JP, Okuja GO, Obonyo R, Latigo MWO (2004) The effect of cassava mosaic geminiviruses on symptom severity, growth, and root yield of a cassava mosaic virus disease‐susceptible cultivar in Uganda. Ann Appl Biol 145(3): 331-337.
- Mohammed IU, Ghosh S, Maruthi MN (2015) Host and virus effects on reversion in cassava affected by cassava brown streak disease. Plant Pathol 65(4): 593-600.
- Tumwegamire S, Kanju E, Legg J, Shirima R, Kombo S, et al. (2018) Exchanging and managing in-vitro elite germplasm to combat cassava brown streak disease (CBSD) and cassava mosaic disease (CMD) in Eastern and Southern Africa. Food Secur 10: 351-368.
- Nweke FI, Spencer DSC, Lynam JK (2002) The Cassava transformation: Africa’s best-kept secret (1st edn), East Lansing: Michigan State University Press, Michigan, US.
- Hillocks RJ, Raya M, Thresh JM (1996) The association between root necrosis and above-ground symptoms of brown streak virus infection of cassava in Southern Tanzania. Int J Pest Manage 42(4): 285-289.
- Gondwe FMT, Mahungu MN, Hillocks RJ, Raya MD, Moyo CC, et al. (2003) Economic losses experienced by small-scale farmers in Malawi due to cassava brown streak virus disease. In: Legg JP, Hillocks RJ (Eds.), Cassava brown streak virus disease: past, present, and future: The Proceedings of an International Workshop, October 27-30, Mombasa, Kenya. pp. 28-36.
- Mtunguja MK, Laswai HS, Muzanila YC, Ndunguru J (2014) Farmer’s knowledge on selection and conservation of cassava (Manihot esculenta) genetic resources in Tanzania. J Biol Agric Health 4(10): 120-129.
- Tumuhimbise R, Melis R, Shanahan P, Kawuki R (2012) Farmers’ perceptions on early storage root bulking in cassava (Manihot esculenta Crantz) in East and Central Uganda and their implication for cassava breeding. World J Agric Sci 8(4): 403-408.
- Danquah AJ, Gracen VE, Offei SK, Asante IK, Aduening JM (2016) Genetic variability in storage root bulking of cassava genotypes under irrigation and no irrigation. Agr Food Sec 5: 9.
- Mugogo SE, Njapuka A (2007) Soil profile description and analytical interpretation at Chambezi and Naliendele in coastal Tanzania. In: Makamilo GS, Tenga E (Eds.), Annual research report 2007/8. Tanzania Agricultural Research Institute-Naliendele (TARI-Naliendele), Mtwara, Tanzania, pp. 56-67.
- Kundy AC, Mkamilo GS, Misangu RN (2014) Assessment and selection of superior genotypes among elite cassava genotypes by farmers and scientists in Southern Tanzania. J Nat Sci Res 4(17): 24-32.
- Hahn SK, ER Terry, K Leuschner (1980) Breeding cassava for resistance to cassava mosaic disease. Euphytica 29: 673-683.
- Kamau J, Melis R, Laing M, Derera J, Shanahan P, et al. (2011) Farmers’ participatory selection for early bulking cassava genotypes in semi-arid Eastern Kenya. J Plant Breed Crop Sci 3(3): 44-52.
- Masinde EA, Ogendo J, Mkamilo G, Maruthi MN, Hillocks R, et al. (2016) Occurrence and estimated losses caused by cassava viruses in Migori County, Kenya. Afr J Agric Res 11(24): 2064-2074.
- Kawano K (1987) Inherent and environmental factors related to cassava varietal selection. In: E. Hershey, editor, Cassava breeding: a multidisciplinary review. Centro Internacional de Agricultura Tropical (CIAT), Cali, Colombia, pp. 207-226.
- SAS (2013) Statistical Analysis System 13.1 for Windows. SAS Institute, Cary, North Carolina.
- Ariyo OA, Dixon AGO, Atiri GI (2003) Effect of detopping on disease incidence and symptom severity of African cassava mosaic virus disease (ACMD) on some newly developed cassava cultivars from landraces introgression. Acta Phytopathol Entomol Hung 38(1-2): 115-124.
- Mohammed IU, Abarshi MM, Muli B, Hillocks RJ, Maruthi MN (2012) The symptom and genetic diversity of cassava brown streak viruses infecting cassava in East Africa. Adv Virol 2012: 795697.
- Chipeta MM (2016) Identification and development of cassava brown streak disease resistant and early storage root bulking varieties in Malawi. University of KwaZulu-Natal, KwaZulu-Natal, South Africa.
- Kulembeka PKH (2009) Genetic linkage mapping of field resistance to cassava brown streak disease (CBSD) in cassava (Manihot esculenta Crantz) landraces from Tanzania. Ph.D. diss. University of the Free State, Bloemfontein.
- Maruthi MN, Bouvaine S, Tufan HA, Mohammed IU, Hillocks RJ (2014) Transcriptional response of virus-infected cassava and identification of putative sources of resistance for cassava brown streak disease. PLoS One 9(5): e0096642.
- Bredeson JV, Lyons JB, Prochnik SE, Wu GA, Ha CM, et al. (2016) Sequencing wild and cultivated cassava and related species reveals extensive interspecific hybridization and genetic diversity. Nat Biotechnol 34: 562-570.
- Masumba EA, Kapinga F, Mkamilo G, Salum K, Kulembeka H, et al. (2017) QTL associated with resistance to cassava brown streak and cassava mosaic diseases in a bi-parental cross of two Tanzanian farmer varieties, Namikonga and Albert. Theor Appl Genet 130(10): 2069-2090.
- Muhanna M, Mtunda K (2003) Cassava brown streak virus disease transmission studies in Tanzania. In: Legg JP, Hillocks RJ (Eds.), Cassava brown streak virus disease: past, present, and future: The Proceedings of an International Workshop, October 27-30, Mombasa, Kenya. pp. 39-40.
- Ferguson ME, Hearne SJ, Close TJ, Wanamaker S, Moskal WA, et al. (2012) Identification, validation and high-throughput genotyping of transcribed gene SNPs in cassava. Theor Appl Genet 124: 685-695.
- Nzuki I, Katari MS, Bredeson JV, Masumba E, Kapinga F, et al. (2017) QTL mapping for pest and disease resistance in cassava and coincidence of some QTL with introgression regions derived from Manihot glaziovii. Front Plant Sci 8: 1168.
- Jennings DL (2003) Historical perspective on breeding for resistance to cassava brown streak virus disease. In: Legg JP, Hillocks RJ (Eds.), Cassava brown streak virus disease: past, present, and future: The Proceedings of an International Workshop, October 27-30, Mombasa, Kenya, Natural Resources International Limited, Aylesford, UK, pp. 55-57.
- Kanju E, Mkamilo G, Mgoo V, Ferguson M (2010) Statistical evidence linking the zigzag stem habit with tolerance to cassava brown streak disease. Roots 12(2): 4-6.
- Pariyo A, Tukamuhabwa P, Baguma Y, Kawuki RS, Alicai T, et al. (2013) Simple sequence repeats (SSR) diversity of cassava in South, East and Central Africa in relation to resistance to cassava brown streak disease. Afr J Biotechnol 12(28): 4453-4464.
- Masinde EA, Mkamilo G, Ogendo J, Hillocks R, Mulwa RMS, et al. (2017) Genotype by environment interactions in identifying cassava (Manihot esculenta Crantz) resistant to cassava brown streak disease. Field Crops Res 215: 39-48.
- Kanju E, Uzokwe VNE, Ntawuruhunga P, Tumwegamire S, Yabeja J, et al. (2019) Varietal response of cassava root yield components and root necrosis from cassava brown streak disease to time of harvesting in Uganda. Crop Prot 120: 58-66.
- Kaweesi T, Kawuki R, Kyaligonza V, Baguma Y, Tusiime G, et al. (2014) Field evaluation of selected cassava genotypes for cassava brown streak disease based on symptom expression and virus load. Virol J 11: 216.
- Anikwe MAN, Ikenganyia EE (2018) Ecophysiology and production principles of cassava (Manihot species) in Southeastern Nigeria. In: Waisundara V (Ed.), Cassava, Intech Open, London, pp. 105-122.
- Jennings DL (1960) Observations on virus disease of cassava in resistant and susceptible varieties. II. Brown streak disease. Empire J Exp Agr 28(111): 261-270.
- Kanju E, Tumwegamire S, Legg J (2017) Elite disease resistant germplasm successfully exchanged and evaluated in five African countries. In: International Institute of Tropical Agriculture (IITA) annual report 2017. IITA, Ibadan, Nigeria, pp. 33-35.
- Tanzania Meteorological Agency (2013) Precipitation, temperature and relative humidity data, 2013. Dar es Salaam: Tanzania Meteorological Agency (TMA), Tanzania.
© 2020 Midatharahally N Maruthi. This is an open access article distributed under the terms of the Creative Commons Attribution License , which permits unrestricted use, distribution, and build upon your work non-commercially.
 a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in
a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in 







.jpg)






























 Editorial Board Registrations
Editorial Board Registrations Submit your Article
Submit your Article Refer a Friend
Refer a Friend Advertise With Us
Advertise With Us
.jpg)






.jpg)














.bmp)
.jpg)
.png)
.jpg)










.jpg)






.png)

.png)



.png)






