- Submissions

Full Text
Modern Concepts & Developments in Agronomy
Stable Isotope Analysis as a Tool to Determine Nitrogen Fertilizer Source
JMarlee A Trandel1*, Penelope Perkins Veazie1 and S Alan Walters2
1Department of Horticultural, USA
2Department of Plant, Soil, and Agricultural Systems, USA
*Corresponding author: Marlee A Trandel, 600 Laureate Way, Plants for Human Health Institute, Department of Horticultural Sciences, North Carolina State University, Kannapolis, NC 28081, USA
Submission: August 02, 2019;Published: August 22, 2019

ISSN 2637-7659Volume5 Issue1
Abstract
Fingerprinting crops to detect organic or inorganic fertilizer use can be done by determining nitrogen (N) stable isotope values. In previous research, crops grown with organic N had higher amounts of 15N while those grown with inorganic N were higher in 14N. This information may be useful to follow plant demands and N requirements of heavy N feeding crops like tomato (Solanum lycopersicum) and lead to more efficient N inputs. A greenhouse experiment was conducted with ‘Better Bush’ tomatoes using four soil fertility treatments consisting of a) inorganic fertilization [Miracle Grow®; 24N-4P-13K], b) organic fertilization [bonemeal (6N-8P-0K), bloodmeal (12N-0P-0K), liquid Earth juice (2N-1P-1K) and 25% vermicompost]; c) mixed fertilization [Miracle Grow® with 25% vermicompost] and d) control [no fertilization].
Plant vigor assessment ratings clearly showed significant differences with the applied N source (i.e., none, organic and inorganic). Nitrogen isotopes of tomato leaflets, fruit peels and juice differed among treatments. The 15N was highest in plants from organic fertilization with vermicompost treatment, lower in those from the mix of inorganic and vermicompost, and absent in tissues from plants grown with inorganic N fertilizer. Nitrogen isotopes can be easily determined from dry plant material for a low monetary cost, offer a means of screening for nitrogen fertilization source, and may assist with the organic certification process. For tomato, dried mature leaflets or fruit peels are recommended for nitrogen stable isotope analysis. Nitrogen isotopes may slightly vary dependent upon tomato varieties grown or with those that have been grafted, and further research needs to compare N isotopic values from different tomato varieties and/or grafting treatments to better highlight N isotopic patterns.
Keywords: Organic agriculture; Organic certification; Tomato; Vermicompost
Introduction
Nitrogen is critical for vegetable crops to maximize productivity, and the assimilation of this nutrient by plants is important because it directly influences plant growth, development and stable N isotopic patterns. Nitrogen fractionates into the stable isotopes of either 14N or 15N, and often depends on the fertilizer source applied to the soil [1,2]. These fractionation events will provide differences in plant isotopic responses which are directly influenced by inorganic or organic fertilizers. Inorganic fertilizers are generally comprised into 14N, the lighter and more abundant form of nitrogen, while organic fertilizers, manures and composts are composed of the heavier form, 15N [1,2]. Inorganic nitrogen fertilizers generally increase the amount of 14N in plant foliage and fruit [3]. Plants fertilized with mixed N forms (inorganic and organic) will have similar 15N/14N isotopic ratios. As the demand for organic crops continue to increase, both organic certification agencies and consumers require assurance that organically labeled products have been grown using organic methods. Organic nitrogen management is fundamental to organic label compliance. Fingerprinting crops to detect organic or inorganic fertilizer use can be done by determining nitrogen (N) stable isotope values. The 15N/14N ratios in corresponding plant parts provide a useful tool to differentiate among plants grown with various N fertilization methods [2]. Therefore, N stable isotope analysis can be used as an objective test to differentiate between organic and inorganic fertilized crops [3]. However, more research is needed to gain a better understanding of 15N/14N isotopic ratios in various vegetable crops grown under different fertilization systems. This information will potentially benefit the organic industry by giving an accurate assessment regarding if the appropriate nitrogen fertilization systems were utilized during production. Thus, a stable N isotope study was initiated to determine the effect of various N fertilizer regimes on tomatoes, since this crop is a heavy nutrient feeder and requires large amounts of N to maximize fruit yields.
Material and Methods
Greenhouse experiment location, setup and materials
A greenhouse study was set up in a randomized complete block design with three replications and repeated over two growing seasons to evaluate the N isotopic compositions of tomato plant leaves, fruit skins and juice and overall plant performance. The experiment was conducted from 10 July to 15 December 2014 and 11 July to 11 December 2015 at the Horticulture Research Center Greenhouse at Southern Illinois University in Carbondale (SIUC), Illinois. ‘Better Boy’ tomatoes were grown in a controlled greenhouse held between 21 to 29 °C to determine the influence of organic and inorganic fertilizers on plant isotopic patterns. Tomato plants were fertilized with the following treatments: a) inorganic fertilization [Miracle Grow®; 24N-4P-13K)], b) organic fertilization [bonemeal (6N-8P-0K), bloodmeal (12N-0P-0K), liquid Earthjuice (2N-1P-1K) and 25% vermicompost], c) mixed fertilization (Miracle Grow® with 25% vermicompost] and d) control (no fertilization applied). Tomatoes, one per pot, were grown in 8 L plastic pots and the base soil consisted of 1:1:1 soil: sand: peat mix, Belnap silt loam (Coarse-silty mixed, mesic Aeirc Fluvquent). Fertilizer amendments were then applied to the soil accordingly where inorganic soil fertility treatments were fertilized with 17g 12-12-12 at the time of soil mixing while those containing vermicompost had 25% vermicompost added at soil mixing. All inorganic treatments were watered bi-weekly with 20g miracle grow diluted in 3.785L water (500ppm N). The organic soil fertility treatments were fertilized with 9g bone meal, 5g blood meal, and 7g potassium sulfate (0-0-50, Great Salt Lakes Minerals Corp, Ogden, UT) at the time of soil mixing. Then they were watered bi-weekly with 36g liquid Earthjuice grow (100ppm N) and 36g liquid Earthjuice bloom (300ppm N) diluted in 7.57L water. The vermicompost was obtained from the SIU Vermicomposting Center which was developed from red wiggler earth worms (Eisenia fetida) feeding on coffee grounds and vegetable wastes. A soil analysis of the 100% vermicompost sample and 1:1:1 soil mix was obtained from Brookside laboratories, New Knoxville, Ohio (Table 1).
Table 1: Soil fertility characteristics of 100% vermicompost and nitrogen fertilization treatments.

Vermicompost=100% vermicompost.
Control=soil not fertilized with any N
Inorganic=positive control, soil fertilized biweekly with Miracle Grow ® (24N-4P-13K)
Organic=soil amended with 25% vermicompost, bone meal (6N-8P-0K), blood meal (12N-0P-0K), and fertilized biweekly with liquid Earthjuice Grow (2N-1P-1K)
Mixed=soil amended with 25% vermicompost, and fertilized biweekly with Miracle Grow ® (24N-4P-13K)
Material and Methods
Isotope ratio mass spectrometer
Nitrogen stable isotopes were measured using an isotope ratio mass spectrometer (IRMS), which measures the isotopic signatures of the sample and reference standard relative to one another. The ‘intensities’ of the sample and the standard were measured in proportion to the abundance of the isotopes collected, and the isotopic ratios or delta values (δ) were automatically calculated by the machine software [3]. Stable isotopes were measured using delta notation (δ), and this expresses their isotopic value measured as parts per thousand, ‰ (i.e., 1/1000). The “δ” and “‰” both mean that samples were measured in parts per thousand, which provides a convenient way of expressing the changes in isotopic values of the samples relative to the standards. Stable isotopes are extremely small values, calculated using the equation below (4):
δ=[(Rsample - Rstandard) / Rstandard] x 1000=‰
A. Rsample=15N/14N ratio of the sample
B. Rstandard=15N/14N ratio of the standard (atmospheric N is the denoted standard)
C. Rsample is subtracted from Rstandard then divided by Rstandard
D. The remaining value is then multiplied by 1,000
Data collection and sample preparation
Tomato leaflets were collected at immature (45d after transplant) and mature (80d after transplant) growth stages. Five tomato leaflets were harvested from each plant and were collected from the tip, middle, and closest to the stem of the topmost branch (branch 1) and from only the very tip of the next two lower branches (branches 2 and 3). Tomato leaflets were placed into sterilized beakers and immediately oven dried. Tomato fruit samples at the red ripe stage were harvested from the first two fruit clusters, with two per cluster immediately placed into separately labeled paper bags and were taken to the laboratory for sample preparation. Sample preparation was done following Trandel et al. [4]. In short, all leaf samples were oven dried for 2 to 3 days at 75 °C then ground with a mortar and pestle to a fine powder and further oven dried for 1 to 2 days at 75 °C. Roughly 1.5 to 3.5mg of leaflet samples was packed into a Costech Analytical 3.5x5mm tin capsule. Tomatoes were prepared by making a circular incision 3 to 5cm in diameter about 0.5cm deep into the tomato skin with scapula and blade. The tomato skin was removed from each fruit and immediately oven dried for 7 to 10 days at 75 °C. The samples were ground into a fine powder and oven dried for an addition 1 to 2 days. For each skin sample, 1.5 to 2mg was packed into a tin capsule. The peeled tomatoes were then placed into large beakers and ground into juice with a large pestle. After macerating the tomatoes, about 35g were placed into 50ml test tubes and were centrifuged at 5,000rpm for 5min. to obtain the supernatant (tomato juice). The resulting juice was filtered through Sterlitech micropore filters and immediately frozen at -18 °C. Just prior to packing in tin capsules, tomato juice samples were thawed, and approximately 0.4ml dropped with a syringe on 20mg of inert Costech Analytical silicon dioxide powder placed into a 5x9mm tin capsules. All samples were analyzed in the isotope ratio mass spectrometer. Tomato plant vigor was rated at experiment termination. Vigor was rated subjectively as 1 to 3 (low), 4 to 6 (moderate), and 7 to 9 (high). Low plant vegetative growth, fruit count and major discoloration was considered low vigor; moderate plant vegetative growth, fruit count and discoloration was medium vigor; and high plant vegetative growth, fruit number and minimal discoloration was high vigor. The harvested tomato plants were placed into large, labeled paper bags and air dried in the greenhouse at 32.2 °C, and weights determined.
Data analysis
Data was analyzed using a two-tailed analysis of variance (ANOVA) (JMP Statistical Discovery Software, JMP, 2014 and 2015 Cary, NC). The least significant difference (LSD) and Student’s t multiple range tests were used to separate fertilizer treatment means at P≤0.05.
Results and Discussion
Nitrogen fertilization and isotopic values
Inorganic fertilizers decreased the amount of 15N in plant parts. In both growing seasons, tomatoes fertilized with inorganic N source (Miracle Grow ®) had the lowest δ15N isotopic values in tomato leaflets ranging from -2.2 to 0.7‰. Tomato leaflets from organic fertilization ranged from 6.5 to 7.1‰ and those from mixed fertilization were 3.2 to 3.4‰. Leaflets from the control treatment were 3.4 to 3.8‰. Tomato peels and fruit juices showed similar N isotope trends among fertilization treatments as the leaflets (Table 2). Plants uptake both ammonium and nitrate with mostly the lighter isotope of 14N being assimilated when inorganic fertilizers are used (Figure 1). Although there are both stable isotopes of 15N and 14N in inorganic fertilizers, the availability of 14N coming from fast release ammonium and nitrate results in little to no fractionation or negative δ15N values in plant tissues [5,6].
Figure 1:Description of plant isotope fractionation and nitrogen discrimination in organic and convention nitrogen fertilization systems (Figure Credit: Dr. Mihai Lefticariu, Southern Illinois University Carbondale, 2015).
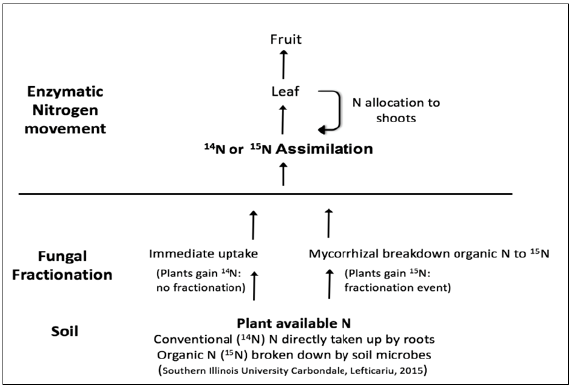
Table 2: Average δ15N values found in the greenhouse tomatoes fertilized with organic, inorganic or mixed nitrogen sources.
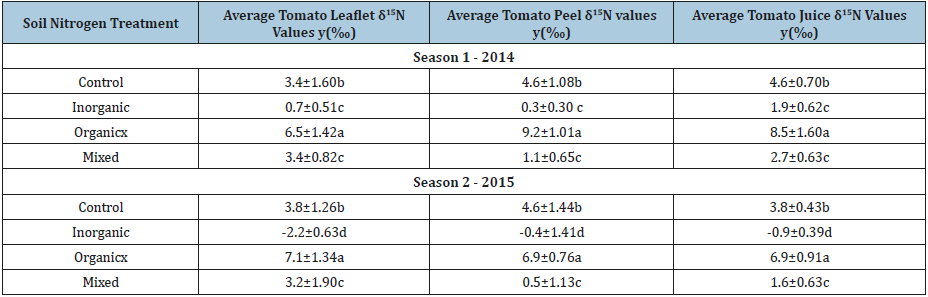
Standard deviations incorporated into isotopic values
xOrganic plant isotopic signatures significantly higher than all other treatments (p-value<0.0001)
yMeans with different letters indicates significant differences based on Fisher’s Least Significant Difference Test at P≤0.05.
Conversely, organic fertilizers go through two fractionation events. Organic N is released through mineralization and enters into a soil ‘pool’ assimilated as organic N [7,8]. This fractionation happens as soil microbes break down organic N and make it available to plant roots as 15N. The organic soils fertilized with ‘Nin” have higher amounts of 15N compared to assimilated “N-out” as microorganisms tend to discriminate and hold onto some of the 15N. Once converted to organic N, the plant product becomes depleted of 14N, causing an increase in 15N values [1,3]. The second fractionation event occurs as N moves from plant roots to shoots, where the heavier isotope is shuttled throughout the plant and results in higher δ15N values (Figure 1).
Nitrogen source influences stable isotope form in tomato plant tissue
In this greenhouse tomato study, a clear increase in 15N was seen with organic fertilizer sources. Plants fertilized with inorganic N had no appreciable 15N accumulation [1]. The lack of discrimination in tomatoes with inorganic N fertilizer suggests that fractionation does not occur with high influxes of 14N due to high-affinity transport mechanisms of both ammonium and nitrate in plant roots and shoots [9,10]. Another impact of the inorganic treatment can be seen in Table 1, regarding the lower amounts of N released from the soil in 2014 and 2015 at 76.0 and 116.5kg/ ha, respectively, compared to other treatments. This indicates that plants opted to uptake ammonia and nitrate provided from weekly liquid applications over soil available N, leading to lower 15N values.
The organic and mixed fertilizer applications with vermicompost had the highest δ15N amounts in tomato leaflets, peel and juice samples (Table 2). Both organic N and vermicompost enrich the 15N isotopic abundance in the soil profile. It is more difficult for living microbes and plants to assimilate 15N, as this form of N is heavier with a slower vibrational energy [2,9]. The addition of vermicompost and other organic N fertilizers to the soil enhance the soil organic matter content, which will directly influence soil fertility characteristics. Both the organic and mixed fertilization treatments had the high amounts of released N, ranging from 109.0 to 177.0kg/ ha over the two years (Table 1). The application of vermicompost and slow release N sources increased the soil microbial activity and the soil’s ability to retain the heavier N isotope, ultimately leading to higher δ15N values in the organic and mixed fertilization treatments (Table 2) [10].
Plants that were not fertilized with any additional N (control treatment) generally had higher δ15N values compared to those containing inorganic N sources. Additionally, the control treatment can still be distinguished from those fertilized with organic N sources, as the 15N isotopic values were 3 to 4‰ lower (Table 2). The increase in δ15N compared to the tomato plants fertilized with inorganic sources probably resulted from the natural N and organic matter present in the soil. The control treatment had low amounts of released N at 67.2 and 118.7kg/ha for 2014 and 2015, respectively (Table 1); and, this organic N had to first by broken down to plant available N before being taken up by the tomato plant. As plants continued to mature and produce fruit, the 15N values slightly decreased probably due the microbes retaining the heavier (15N) isotope and allowing for the lighter (14N) isotope to be completely up taken from the soil during plant and fruit maturation.
Nitrogen application effects on tomato vigor
Tomato plant vigor and growth differed in response to the fertilizer programs applied (Table 3), with observed nutrient deficiencies shown in Figure 2. Tomato plants with no fertilization were lowest in vigor ratings and dry weight. Discoloration was exhibited as interveinal chlorosis, yellowing and purple on the leaves. Tomatoes fertilized with only organic N sources displayed moderate to high vigor, moderate amounts of biomass and minimal chlorosis and purple discoloration. The tomato plants fertilized with inorganic or mixed fertilizer (inorganic plus 25% vermicompost) had high vigor, high biomass and no discoloration. Blossom end rot was observed only on tomato fruit grown with inorganic N sources. Plant vigor assessments help address the variability in plant responses to N fertility applications and isotopic values. The organic treatment with moderate amounts of vigor can be potentially related to the heavier 15N isotope found in organic fertilization sources [2]. Although the organic and mixed treatments had the highest amount of N release (Table 1), organic N sources are heavier due to the addition of one proton (15N=heavier, 14N=lighter) with slower vibrational energies leading to lower vigor responses. Tomato fruit from plants grown with inorganic N indicated the highest plant vigor responses (Table 3). This is due to the availability of inorganic ammonium and nitrate, providing the plants with the lighter 14N. Although there was released N identified in the soil characteristics, the tomatoes preferentially uptake forms of N with high affinity transport mechanisms as this form is easier to assimilate. Supplementation with either vermicompost or organic fertilizers may decrease blossom end rot as increased soil organic matter tends to increase the selectivity of calcium over ammonium ions [11].
Figure 2:Tomato plant vigor and nutrient deficiency responses to fertility treatments evaluated in the second year of the greenhouse study. Control=no added fertilizer,; Inorganic=fertilized with Miracle Grow ® (24N-4P- 13K); Organic=25% vermicompost, bone meal (6N-8P-0K blood meal (12N-0P-0K), and fertilized biweekly with liquid Earthjuice Grow (2N-1P-1K); Mixed=25% vermicompost, and fertilized biweekly with Miracle Grow ® (24N-4P-13K) (Photo Credit: Marlee A Trandel, 2015).
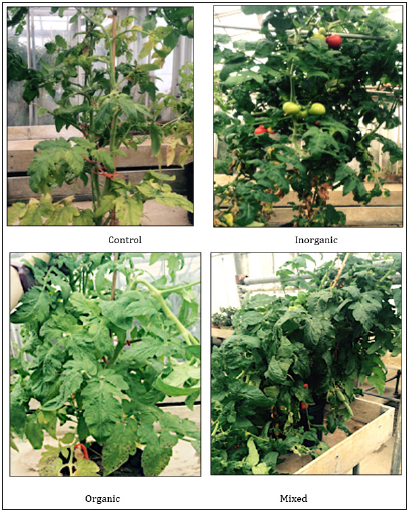
Table 3: Tomato plant vigor and dry weight responses based on fertilization with organic, inorganic or mixed nitrogen sources combined over 2014 and 2015 growing seasons.
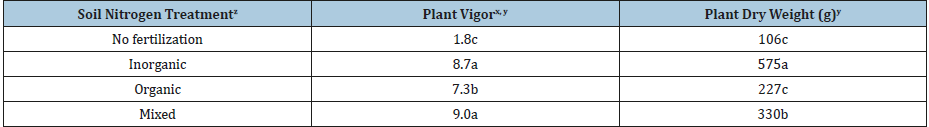
z =See Table 1 for description of treatments.
xPlant vigor was rated as 1 to 3 (low); 4 to 6 (moderate); and 7 to 9 (high). Low vigor represented little plant vegetative growth, few fruit and severe leaf discoloration; medium vigor represented moderate plant vegetative growth, fruit count and leaf discoloration; and high vigor represented high plant vegetative growth, fruit number and minimal leaf discoloration.
yMeans with different letters indicates significant differences based on Fisher’s Least Significant Difference Test at P≤0.05.
Vermicompost benefits and effect on nitrogen isotopes
Vermicomposting increases nutrient mineralization rates by actively decomposing unavailable soil organic matter into stabilized soil aggregates and residues [12]. These aggregates provide food to microorganisms which further convert the residues into a humus-like substances having a finer structure than that of ordinary compost. Humus is largely related to the soil’s ability to retain nutrients and water. Humus supplies organic chemicals to the soil pool that can serve as chelates, which in turn hang onto trace elements and increase their availability to plants. In general, vermicomposting results in a stable organic product that has a high N content and provides other nutrients such as phosphorus, magnesium, and calcium. In this study, δ15N values increased in tomatoes supplied with vermicompost, similar to reports with organic fertilizers [13,14].
Nitrogen metabolism in tomato varieties or with grafting Nitrogen uptake, partitioning and metabolism can vary dependent upon tomato variety or use of grafting. Tomato scions grafted to tomato rootstocks have been heavily introduced into the market as a means to increase fruit quality and decrease plant disease issues [15]. Tomatoes store considerable quantities of nitrate in the vacuoles of root cells which are then metabolized and shuttled to the shoots. Grafting may affect N transport mechanisms due to changes in hydraulic conductance and turgor pressure [16]. Nitrogen transport can be affected by numerous mechanisms both in soil and during assimilation in plants. A similar idea can be seen in various tomato varieties, as different tomato types metabolize and assimilate N at different rates [16]. These changes in N metabolism may ultimately change plant N isotopic patterns and must be considered when using stable isotope analysis as a means to differentiate organic from inorganic tomatoes.
Greenhouse compared to field production and nitrogen isotopes
Organic fertilization regimes that use composts have a higher abundance of 15N, which results in plant tissues more enriched in 15N. This occurs mainly from preferential uptake forming a higher 15N/14N ratio. The use of isotopic techniques in quantifying 15N or 14N values either in tomato fruit under field conditions or in greenhouse studies resulted in similar trends. This suggests that isotopic analysis can be used in both types of production systems as a means to differentiate fertilization treatments.
Summary
This study demonstrated that tomato plants supplied with inorganic N differ in stable isotope values compared to those grown with vermicompost and organic N sources. This study also indicates that mixed N systems have 15N values 3 to 4‰ lower when compared directly to those fertilized with only organic N. Nitrogen isotopic values alongside vigor assessments can be used as a means to identify various fertilization applications and how they affect plant health and fruit yields. Nitrogen stable isotope analysis is an applicable test for organic certification. Isotopic values of mature plant organs can alert certification agencies as to whether heavy nutrient feeding crops, like tomato, have been grown using organic or inorganic fertilization methods. Organic certification agencies implement several rules regarding fertilization and this study emphasized that N isotopes can distinguish if a crop has been grown using the correct N fertilizers. Nitrogen isotopic analysis can be implemented at the wholesale market level to ensure organically labeled crops have been grown correctly. Certification agencies can also start to incorporate the crop’s isotopic value in labels as a means ensure consumers that their food crops are organic. It is important to consider that N isotopic signatures can slightly vary dependent upon the variety or cultivar used and/or the production soil type, even with heavy N-feeding crops. Further investigation may be useful to compare various tomato cultivars, grafting treatments and/or growing conditions to better emphasize differences in 15N values.
Acknowledgement
This work was supported by the USDA Specialty Crop Block Grant Program, Illinois Department of Agriculture, award number: SC16-09.
References
- Bateman A, Simon K, Woolfe M (2007) Nitrogen isotopic compositions of organically and conventionally grown crops. J Agric Food Chem 55(7): 2664-2670.
- Zhou W, Chun Sheng H, Ji L, Christie P, Xiao Tang J (2013) Natural δ15N abundance of winter wheat and soil amended with urea and compost: a long-term experiment. J Food Agric Environ 23: 835-843.
- Evans R D (2001) Physiological mechanisms influencing plant nitrogen isotope composition. Trends Plant Sci 6(3):121-126.
- Trandel MA, Vigardt A, Walters SA, Lefticariu M (2018) Nitrogen isotope composition, nitrogen amount and fruit yield of tomato plants affected by the soil-fertilizer types. ACS Omega 3(6): 6419-6426.
- Sharp Z (2007) Principles of stable isotope geochemistry. Pearson Prentice Hall, Upper Saddle River, USA, p. 344.
- Alderfasi AA, (2010) Prospective study of using bio-organic farming system on growth, nitrate, oxalate and ascorbic acid contents. World Appl Sci J 9(1): 49-54.
- Handley LL (1992) Shoot nitrogen isotope correlates with genotype and salt stress in C3 Planta 201(1): 100-102.
- Arditi R, Ponsard S (2001) Detecting omnivory with δ15N. Trends Ecol Evol 16(1): 20-21.
- Orozco FH (1995) Vermicomposting of coffee pulp using the earthworm Eisenia fetida: Effects on C and N contents and the availability of nutrients. Biol Fertil Soils 22(1-2): 162-166.
- Uchida T, Kaneko N, Ito MT, Futagami K, Saki TS, et al. (2004) Analysis of the feeding ecology of earthworms (Megascolecidae) in Japanese forests using gut content fractionation and δ15N and δ13C stable isotope natural abundances. Applied Soil Ecology 27(2): 153-163.
- Chung J, Zasoski RJ (1994) Ammonium-potassium and ammonium-calcium exchange equilibria in bulk and rhizosphere soil. Soil Soc Am J 58(5): 1368-1375.
- Zucco MA, Walters SA, Chong SK, Klubek BP, Masabni JG (2015) Effect of soil type and vermicompost applications on tomato growth. International J Recycled Organic Waste Agric 4(2): 135-141.
- Christie P, Zhou W, Hu C, Li J, Ju X (2012) Natural δ15N abundance of tomato and soil amended with urea and compost. J Food Agric Environ 10(1): 287-293.
- Dominquez J, Sampedro L (2008) Stable isotope natural abundances (δ13C and δ15N) of the earthworm Eisenia fetida and other soil fauna living in two different vermicomposting environments. Appl Soil Ecol 38(2): 91-99.
- Rivard CL, O Connell S, Peet MM, Welker RM, Lous F (2012) Grafting Tomato to Manage Bacterial Wilt Caused by Ralstonia solanacearum in the Southeastern United States. Plant Dis 96(7): 973-978.
- Asins MJ, Albacete A, Andujar CM, Alfocea FP, Dodd IC, et al. (2017) Genetic analysis of rootstock-mediated nitrogen (N) uptake and root-to-shoot signaling at contrasting N availabilities in tomato. Plant Sci 263: 94-106.
© 2019 Cristina Castillo. This is an open access article distributed under the terms of the Creative Commons Attribution License , which permits unrestricted use, distribution, and build upon your work non-commercially.
 a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in
a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in 







.jpg)






























 Editorial Board Registrations
Editorial Board Registrations Submit your Article
Submit your Article Refer a Friend
Refer a Friend Advertise With Us
Advertise With Us
.jpg)






.jpg)














.bmp)
.jpg)
.png)
.jpg)










.jpg)






.png)

.png)



.png)






