- Submissions

Full Text
Modern Concepts & Developments in Agronomy
Removal of Cr (VI) Through the Use of the Agroindustrial Residue of the Persea Americana Shell
Rodríguez A1, López VH1, Nájera Pérez MM1, Cárdenas González JF2*, Rodríguez Pérez AS2, Martínez Juárez VM3 and Michel Cuello C2
1Faculty of Chemical Sciences, Laboratory of Experimental Mycology, Mexico
2Multidisciplinary Academic Unit Middle Zone, Mexico
3Academic Area of Veterinary Medicine and Zootechnics, Mexico
*Corresponding author: Cárdenas González JF, Multidisciplinary Academic Unit Middle Zone, Mexico
Submission: December 14, 2018;Published: January 22, 2019

ISSN: 2637-7659 Volume3 Issue4
Abstract
We analyzed the Chromium (VI) removal capacity in aqueous solution by the Persea americana biomass, using the diphenyl carbazide method to evaluate the metal concentration. Biosorption at different pH (1, 2, 3, and 4) was evaluate for different times. We too studied the effect of temperature in the range of 28 to 60 °C and the removal at different initial concentrations of Cr (VI) of 200 to 1000mg/L. Therefore, the highest biosorption of the metal (50mg/L) occurs within 270 minutes, at pH of 1.0 and 28 °C. According to temperature, the highest removal was observing at 60 °C, in 45 minutes, when the metal is completely adsorbed. At the analyzed concentrations of Cr (VI), fungal biomass, showed excellent removal capacity, besides it removes efficiently the metal in situ (100% removal in earth and water contaminated, after 5 and 6 days of incubation, 5 and 10g of biomass, 10g of earth and 100mL of water; so, it can be used to eliminate it from industrial wastewater.
Keywords: Chromium (VI); Removal; Shell biomass; Detoxification
Introduction
Currently, one of the main environmental problems is heavy metal pollution. These elements alter the equilibrium of ecosystems by persisting indefinitely in the environment, because they are not degrading by biological or chemical means [1]. Its accumulation in the organisms of the different links of the trophic chain, its mobility in natural aquatic ecosystems and its toxicity, make its elimination a global concern [2]. The introduction and redistribution of metal ions in the biosphere have their origin from natural and anthropogenic sources [3]. However, the main direct as well as indirect cause of the metal contamination are the urban sources, being the industrial operations with a deficient or absent treatment of its residual waters and solid waste, its main emitter. Some of the industries that generate waste contaminated with these elements are sugar, oil, brewing, textiles, cellulose and paper, metal finishing, copper and its alloys, tannery, food and of iron and steel, legally classified as point sources of pollution [2]. Hexavalent chromium [Cr (VI)] is an important water pollutant. Even at Cr (VI) levels measuring in the parts per billion (ppb), research has shown it to be toxic [4]. Cr (VI) can originate from different anthropogenic activities such as chromite mining, leather tanning, pigment synthesis, electroplating and metal finishing [4]. The primary forms of chromium found in nature are chromium (III) and chromium (VI) and these forms are convert to each other depending on environmental conditions [5]. Cr (VI) is consider the most toxic form of chromium is usually associated with oxygen as chromates (CrO4-2) and dichromate (Cr2O7 -2) [4].
It has been established now that various chromium compounds as oxides, chromates and dichromate, are environmental contaminants in water, soil, and industrial effluents, because this metal is widely used in various manufacturing, such as electrolytic plating, explosives manufacturing, leather tanning, metal alloy, dyes and pigments manufacturing, etc., [2,3,5]. There are studies of the current technologies that are being used to effectively reduce Cr (VI) present in aqueous solutions by means of chemical, electrochemical and biological methods, for example: ion exchange on resins, coagulation-flocculation, adsorption on activated carbon, reduction, chemical precipitation, sedimentation, etc., [6], which in most cases are expensive or inefficient, especially when the concentration of these ions is very low [7]. Therefore, arise emerging technologies such as biosorption, the process of attracting various chemical species by biomass (live or dead), by physicochemical mechanisms as adsorption or ion exchange [8]. Recently, varieties of low cost materials have been study for their ability to remove Cr (VI) from aqueous solution and promising results are shown. Among these low cost adsorbents are dead microorganisms, clay minerals, agricultural wastes, industrial wastes and various other low cost materials [4,6-9]. Thus, there is a need to develop or find innovative low cost adsorbents with an affinity towards metal ions for the removal of Cr (VI) from aqueous solution, which leads to high adsorption capacity [4,6-8]. The objective of this study was to analyze in vitro biosorption of Cr (VI) by Persea americana shell biomass.
Materials and Methods
Biosorbent used
The Persea americana shell, was obtained from the fruits harvested and offered in the market place Republic, between the months of August to September in 2017, of the capital city of San Luis Potosí, S.L.P. México. To obtain the biomass, the shells was washed with tri de-ionized water during 72 hours at constant stirring, with water changes every 12 hours. Subsequently, it was boil 1 hour to removal traces of the fruit and dust and were dry at 80 °C for 12 hours in an oven, ground in blender and stored in amber vials until use.
Biosorption studies and determination of hexavalent, trivalent, and total chromium
For these studies, was used 1g of dried biomass mixed with 100mL of tri deionized water containing 50mg/L in an Erlenmeyer flask at the desired temperature and pH. The flasks were agitated on a shaking bath Yamato BT-25 model at different times. Samples of 5mL were taken at different times and centrifuged at 3000rpm for 5min. The supernatant liquid was separated and analyzed for Cr (VI) ions, which were quantifying by a Spectrophotometric method employing Diphenylcarbazide [10]. The information shown in the results section are the mean from three experiments carried out by triplicate.
Results and Discussion
Effect of incubation time and pH
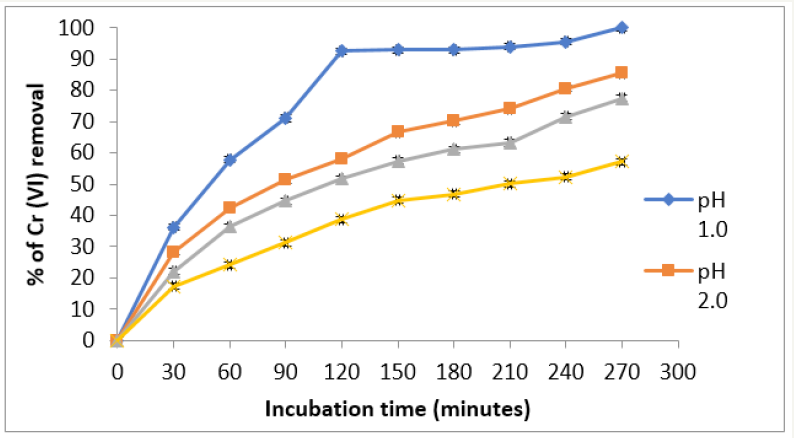
The optimum time and pH for Cr (VI) removal for P. americana was 270 minutes and pH 1.0, at constant values of biosorbent dosage (1g/100 mL), with an initial metal concentration of 50mg/L, and a temperature of 28 °C (Figure 1). It was used a pH meter Corning Pinnacle 530 model and we use nitric acid 1M to maintain the pH. The literature [11], report an optimum time of 4 hours for the removal of Cr(VI) by Amarantus caudatus, with 5g of biomass, 180 minutes for the Cucumis melo shell [12], 9 hours for sawdust of pine tree [13], 16, 60, 120, 180, and 480 minutes, respectively for Magnifera indica, Magnifera paradisiaca, Cucumis paradise,< Cucumis melo, and Cucumis máxima shells [14,15] and pH 1.0, at constant values of biosorbent dosage (1g/100 mL), with an initial metal concentration of 50mg/L. Changes in the cell permeability of unknown origin, could partly explain the differences founded in the incubation time, providing greater or lesser exposure of the functional groups of the cell wall of the biomass analyzed [1,3,6-8]. Adsorption efficiency of Cr (VI) was observe a maximum at pH 1.0 with the biomass analyzed, and this is like to most reports [1,11- 15]. This was due to the dominant species (CrO42- and Cr2O72-) of Cr ions in solution, which were expecting to interact more strongly with the ligands carrying positive charges [1,7,8].
Figure 1:Effect of incubation time and pH on Chromium (VI) removal by the biomass of P. americana shell. 50mg/L Cr (VI), 100rpm, 28 °C.
Effect of the temperature
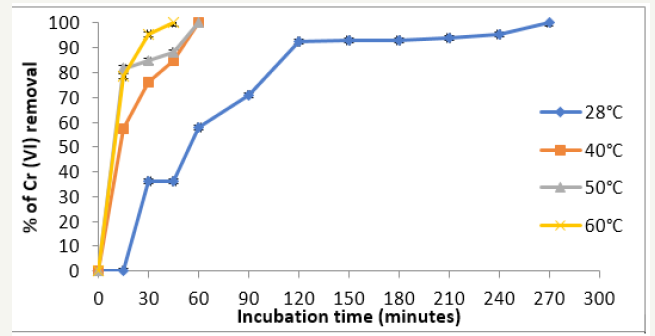
Temperature was found to be a critical parameter in the bio adsorption of Cr (VI) (Figure 2). To maintain constant the temperature in all experiments, we use a shaking bath Yamato BT- 25 model. The total removal was observed at 60 °C and 28 °C with 45 minutes and 64 hours of incubation. This results are coincident for tamarind shell with 95% of removal at 58 °C and 3 hours [16], for the adsorption of cadmium (II) from aqueous solution on natural and oxidized corncob (40 °C and 5 days) [17], but these are different for the mandarin waste [18], Caladium bicolor (wild cocoyam) biomass [19], and Saccharomyces cerevisiae [20]. The increase in temperature increases the rate of removal of Chromium (VI) and decrease the contact time required for complete removal of the metal, to increase the redox reaction rate [16].
Figure 2:Effect of the temperature on Chromium (VI) removal by the biomass of P. americana shell, 50mg/L Cr (VI), pH 1.0, 100rpm.
Effect of initial metal concentration
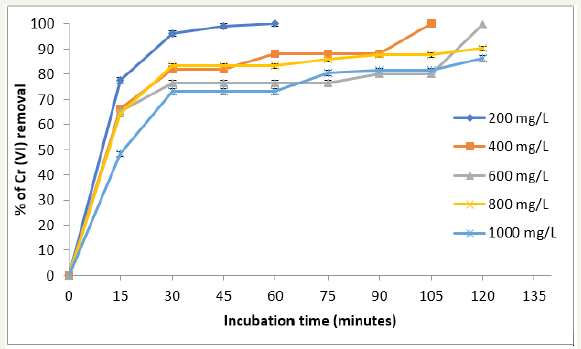
At low metal concentrations (200mg/L), we observe the best results for removal, with the biomass analyzed, 28 °C, because the removal of the metal was 100% at 20 and 64 hours for 200 and 1000mg/L, respectively (Figure 3). In addition, we observe the development of a blue-green and white precipitate, which changes more rapidly at higher temperatures (date not shown). The results are coincident for C. reticulata and Tamarindus indica shell [12,16]. With respect to other biomasses, most authors report lower removal efficiencies of metal, for example: 45mg/L for eucalyptus bark [21], 13.4 and 17.2mg/L for bagasse and sugar cane pulp, 29mg/L coconut fibers, 8.66mg/L for wool [22], 25 and 250mg/L of chitin and chitosan [23], and 1mg/L for cellulose acetate [24]. The increase in initial concentration of Cr (VI), results in the increased uptake capacity and decreased in the percentage of removal of the metal. This was due to the increase in the number of ions competing for the available functional groups on the surface of biomass [16]. On the other hand, at 60 °C, 200 and 1000mg/L, they are eliminated at 60 and 120 minutes, respectively (Figure 4), which is similar to other reports in the literature, for the removal of the same metal concentration by different natural biomasses [11-16].
Figure 3:Effect of initial metal concentration on Cr (VI) removal by biomass of P. americana shell. pH 1.0, 100rpm, 28 °C.

Figure 4:Effect of initial metal concentration on Cr (VI) removal by biomass of P. americana shell. pH 1.0, 100rpm, 60 °C.
Effect of bio sorbent dose
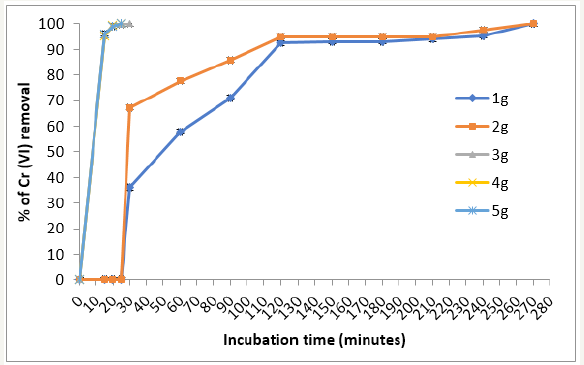
The influence of biomass concentration on the removal capacity of Cr (VI) is depict in Figure 5. If we increase, the amount of biomass also increases the removal of the metal in solution, with more biosorption sites of the same, because the amount of added bio sorbent determines the number of binding sites available for metal biosorption [25]. Similar results have been reported for modified corn stalks [26], C. reticulata shell [27], and Mucor hiemalis and Rhizopus nigricans, although latter with 10g of biomass [18,28], but they are different from those reported for wastes biomass of mandarin (bagasse), with an optimal concentration of biomass of 100mg/L [29].
Figure 5:Effect of biomass concentration of P. americana on the removal of 50mg/L Cr (VI), 28 °C, pH 1.0, 100rpm.
Removal of Cr (VI) in industrial wastes with biomass of P. americana shell
Figure 6:Removal of Cr (VI) in industrial wastes incubated with 5 and 10g of P. americana biomass. 28 °C, 100rpm, 100mL of contaminated water, (50mg Cr (VI)/L (adjusted).
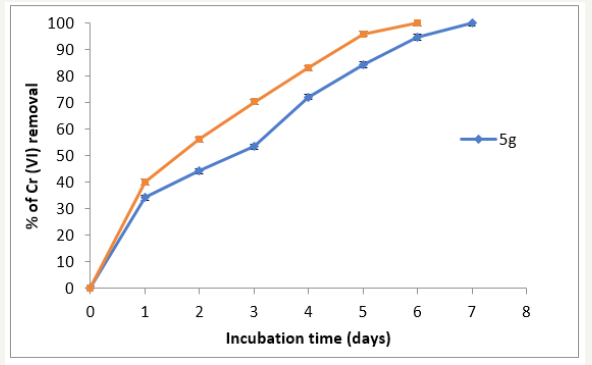
Figure 7:Removal of Cr (VI) in industrial wastes incubated with 5 and 10g of P. americana biomass. 28 °C, 100rpm, 10g of contaminated earth, (50mg Cr (VI)/g earth.
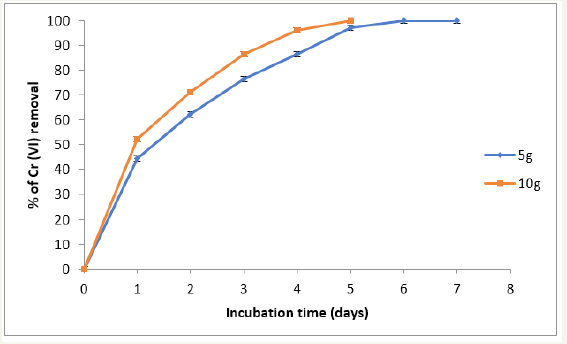
We adapted a water-phase bioremediation assay to explore possible usefulness of this biomass for eliminating Cr (VI) from industrial wastes, the biomass (5 and 10g), was incubate with non-sterilized contaminated earth and water containing 50mg Cr (VI)/g and 50mg Cr(VI)/L (adjusted), suspended in tri deionized water to a final volume of 100mL. It was observing that in 5 and 6 days of incubation with the biomass, the Cr (VI) concentration of earth and water samples decrease fully in both (Figures 6 & 7), and the decrease level occurred without change significant in total Chromium content during the experiments. In the experiment carried out in the absence of the biomass, the Cr (VI) concentration of the earth samples decreased by about of 18% (date not shown); this might be caused by indigenous microflora and (or) reducing components present in the soil [1,4,9,11]. The chromium removal abilities of this biomass are equal or better than those of other reported, for example T. indica, M. paradisiaca, C. limonium, and C. sinensis shells [30], maize leaf [31], C. melo shell [12], sawdust of pine tree [13], M. indica, M. paradisiaca, C. paradise, C. melo y C. máxima shells [14,15].
Conclusion
The biomass analyzed, showed complete capacity of biosorption of 1.0g/L Cr (VI) in solution at different time of incubation, at 28 °C, 100rpm with 1g of biomass, besides this removal the metal in situ (5 and 6 days of incubation, with 5 and 10g of biomass), in earth and water contaminated. These results suggest their potential applicability for the remediation of Cr (VI) from polluted soils in the fields.
Acknowledgement
All the authors are grateful for the cooperation of the Research and Linking Center “El Balandran”, which is an extension of the Multidisciplinary Academic Unit of the UASLP, for the elaboration of some experiments in its facilities. In the same way the authors thank the Faculty of Chemical Sciences of the UASLP for the use of their facilities for the elaboration of some experiments. The authors declare that there is no conflict of interest and that the project was not financed by any instance, it was carried out with their own means.
References
- Ahemad M (2014) Bacterial mechanisms for Cr (VI) resistance and reduction: an overview and recent advances. Folia Microbiol 59(4): 321- 332.
- Chowdhury S, Jafar MA, Al Atta O, Husain T (2016) Heavy metals in drinking water: Occurrences, implications and future needs in developing countries. Sci Total Environ 569-570: 476-488.
- Pérez BL, Salgado BI, Larrea DC, Martínez SA, Cruz AME, et al. (2018) Biosorción microbiana de metales pesados: características del proceso. Rev Cubana de Ciencias Biologicas 6(1): 1-12.
- Pradhan D, Sukla LB, Sawyer M, Rahman PKSM (2017) Recent bio reduction of hexavalent chromium in wastewater treatment: A review. J Ind Eng Chem 55: 1-20.
- US Environmental Protection Agency (1998) Toxicological review of hexavalent chromium. National Center for Environmental Assessment, Office of Research and Development, Washington, DC, USA.
- Barrera DCE, Lugo LV, Bilyeu B (2012) A review of chemical, electrochemical and biological methods for aqueous Cr (VI) reduction. J Hazard Mat 223-224: 1-12.
- Tejada TC, Villabona OA, Garcés JL (2015) Adsorción de metales pesados en aguas residuales usando materiales de origen biológico. Tecno Lógicas 18(34): 109-123.
- Gutiérrez CJF, Romo RP, Santos EF, Espino SaAE, Hernández EH (2016) Microbial interactions with chromium: Basic biological process and applications in environmental biotechnology. World J Microbiol Biotechnol 32(12): 191.
- Acosta RI, Cárdenas GJF, Moctezuma ZMG, Martínez JVM (2013) Removal of hexavalent chromium from solutions and contaminated sites by different natural biomasses. In: Applied bioremediation-active and passive approaches, (Eds) Yogesh B Patil, Prakash Rao, Croatia, pp. 207- 224.
- Greenberg AE, Clesceri LS, Eaton AD (1992) Standard methods for the examination of water and wastewater. American Public Health Association, Washington, DC, USA.
- Rodríguez A, Pacheco NC, Cárdenas JF, Tovar J, Martínez VM, et al. (2017) Bioadsorción de Cromo (VI) en solución acuosa por la biomasa de Amaranto (Amaranthus caudatus). Av Cien Ing 8(2): 11-20.
- Acosta RI, Cárdenas GJF, Moctezuma ZMG, Tovar OJ, Acosta NMZ et al. (2015) Biosorption of Chromium (VI) by Cucumis melo Shell. J Multidiscip Eng Sci Technol 2(5): 988-993.
- Acosta RI, Ruíz TKC, Cárdenas GJF, Moctezuma ZMG, Martínez JVM (2015) Chromium (VI) removal by Sawdust of pine tree. Int J Latest Res Sci Technol 4(2): 124-128.
- Acosta RI, Cárdenas GJF, Torre BME, González EA, Guerrero MSE, et al. (2015) Biosorption of chromium (VI) by different natural biomasses. J Multidiscip Eng Sci Technol 2(7): 1736-1741.
- Alcaraz VI, Torres RL, Cárdenas GJF, Moctezuma ZMG, Martínez JVM, et al. (2015) El uso de la cáscara de toronja (Citrus paradisi), para la eliminación de Cromo (VI) de sitios contaminados. En: Tendencias de Investigación en Ciencias Naturales y Exactas. Editores: Candy Carranza Álvarez y Alejandro Hernández Morales. UASLP 2: 13-20.
- Agarwal GS, Kumar H, Chaudari S (2006) Biosorption of aqueous chromium (VI) by Tamarindus indica seeds. Bioresour Technol 97(7): 949-956.
- Leyva R, Bernal LA, Acosta I (2005) Adsorption of cadmium (II) from aqueous solution on natural and oxidized corncob. Sep Purif Technol 45(1): 41-49.
- Zubair A, Bhatti HN, Hanif MA, Shafqat F (2008) Kinetic and equilibrium modeling for Cr(III) and Cr(VI) removal from aqueous solutions by Citrus reticulate waste biomass. Water, Air, Soil Pollution 191(1-4): 305-318.
- Jnr MH, Spiff AI (2005) Effects of temperature on the sorption of Pb2+ and Cd2+ from aqueous solution by Caladium bicolor (wild cocoyam) biomass. Electron J Biotechnol 8(2): 162-169.
- Ozer A, Ozer D (2003) Comparative study of the biosorption of Pb (II), Ni (II) and Cr (VI) ions onto Saccharomyces cerevisiae: Determination of biosorption heats. J Hazard Mater 100(1-3): 219-229.
- Sarin V, Pant KK (2006) Removal of chromium from industrial waste by using eucalyptus bark. Bioresour Technol 97(1): 15-20.
- Dakiky MM, Khamis M, Manassra A, Mereb M (2002) Selective adsorption of chromium (VI) in industrial wastewater using low-cost abundantly available adsorbents. Adv Environ Res 6(4): 533-540.
- Sag Y, Aktay Y (2002) Kinetic studies on sorption of Cr (VI) and Cu (II) ions by chitin, chitosan and Rhizopus arrhizus. Biochem Eng J 12(2): 143- 153.
- Arthanareeswaran G, Thanikaivelan P, Jaya N, Mohan D, Raajenthiren M (2002) Removal of chromium from aqueous solution using cellulose acetate and sulfonated poly (ether ketone) blend ultrafiltration membranes. Biochem Eng J 139(1): 44-49.
- Cervantes C, Campos J, Devars S, Gutiérrez F, Loza H, et al. (2001) Interactions of chromium with microorganisms and plants. FEMS Microbiol Rev 25(3): 335-347.
- Chen S, Yue Q, Gao B, Li Q, Xu X (2011) Removal of Cr (VI) from aqueous solution using modified corn stalks: Characteristic, equilibrium, kinetic and thermodynamic study. Chem Eng J 168(2): 909- 917.
- Tewari N, Vasudevan P, Guha B (2005) Study on biosorption of Cr(VI) by Mucor hiemalis. Biochem Eng J 23(2): 185-192.
- Bai RS, Abraham TE (2001) Biosorption of Chromium (VI) from Aqueous Solution by Rhizopus nigricans. Bioresour Technol 79(1): 73-81.
- Pantaler RP, Pulyaeva IV (1985) A spectrophotometric study of complexation between chromium and chromazurol S. J Anal Chem (Moscow) 40: 1634-1639.
- Kelly VK, Cerro LM, Reyna Téllez S, Bandala ER, Sánchez SJL (2012) Biosorption of heavy metals in polluted water, using different waste fruit cortex. Phys Chem Earth Parts A/B/C 37-39: 26-29.
- Adesola NA, Oyebamiji J, Adebowale S (2006) Biosorption of lead ions from aqueous solution by maize leaf. Int J Phys Sci 1(1): 23-26.
© 2019 Cárdenas González JF. This is an open access article distributed under the terms of the Creative Commons Attribution License , which permits unrestricted use, distribution, and build upon your work non-commercially.
 a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in
a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in 







.jpg)






























 Editorial Board Registrations
Editorial Board Registrations Submit your Article
Submit your Article Refer a Friend
Refer a Friend Advertise With Us
Advertise With Us
.jpg)






.jpg)














.bmp)
.jpg)
.png)
.jpg)










.jpg)






.png)

.png)



.png)






