- Submissions

Full Text
Modern Applications in Pharmacy & Pharmacology
Potential Use of the Mitomycin Analogue BMY25282 as a Superior Hypoxia Targeted Anticancer Agent
Philip G Penketh1* and William F Hodnick2
1Department of Pharmacology, Yale University School of Medicine, New Haven, CT 06520, USA
2Facet Life Sciences, Sparks, NV, 89434, USA
*Corresponding author: Philip G Penketh, Department of Pharmacology, Yale University School of Medicine, New Haven, CT 06520, USA
Submission: March 3, 2023;Published: March 10, 2023

ISSN 2637-7756Volume3 Issue2
Hypoxia as a Therapeutic Target
Hypoxia can be considered a tumor selective therapeutic target. In normal tissue mean intercapillary distances are approximately 80μm, thus most cells reside ≤40μm from a functional capillary [1]; but in tumor tissues mean functional intercapillary distances >300μm have been reported [1]. The metabolism of O2 is higher than all other nutrients but its free concentrations within capillaries are low (15-40μM). Thus, O2 has an unusually short diffusion range within tissues of <100μm [2,3]. This range is insufficient to cope with the atypical microvasculature of tumors, creating Hypoxic Tumor Regions (HTRs) [4,5]. These HTRs are interspersed on a microscopic scale between relatively normoxic regions lying adjacent to functional capillaries. Although HTRs (0-5μM O2) have impaired vascular delivery, access by small molecules that lack the unusual supply/demand limitations of O2 readily occurs. Therefore, glucose, present at more than 200-fold greater concentrations than O2, is not limiting and can sustain HTR cells despite their elevated glycolytic rates. HTRs are a major factor in therapy resistance and disease progression and are a strongly negative prognostic factor [1-5]. Radiation can be used to precisely target tumor tissues, but the sensitivity of cells to radiation is greatly diminished in the absence of O2, thus HTR cells tend to survive radiotherapy [4,6]. Additionally, cancer stem cells that represent the clonogenic core of many tumors, appear to preferentially proliferate and reside in HTRs [7]. These, like normal stems cells, are equipped for long term survival, possessing high levels of enzymes involved in detoxification and self-maintenance, [7] making them more resistant to chemotherapy. Thus, cells in HTRs are more likely to survive radiotherapeutic and chemotherapeutic treatments, cause relapse, and ultimately lead to patient death. Strategies to deliver a stronger cytotoxic blow to therapy-resistant HTRs are therefore required. Cells within HTRs exhibit considerably more net reduction of xenobiotics containing particular functional groups, largely due to decreased back oxidation by O2 [4,8].
Mitomycins: A Nature-Designed Hypoxia Selective Cytotoxin
Mitomycin C (MC) and Porfiromycin (POR) are antibiotic products of Gram-positive Streptomyces soil bacteria. The role of these compounds is to kill competing soil bacteria. Mitomycins can be reduced in two distinct manners, one electron reduction to yield the semiquinone radical anion, and two electron reduction to generate the hydroquinone. The one-electron reduction product, the mitomycin semiquinone radical anion (MC∙-) [9], reacts with molecular oxygen (itself a stable diradical) at close to the diffusion controlled rate of 109-1010 M-1s-1) to regenerate the parental mitomycin quinone and superoxide (O2∙-) [10,11]. Since very few cross-links are required to give rise to a lethal event [10,11], the regeneration of MC and the production of a O2∙-, represents a detoxification step. Under physiological O2 concentrations, the half-life of MC∙- would be expected to be less than 0.1ms. Therefore, in the presence of physiological tissue concentrations of oxygen (~20μM), only an extremely small proportion of the MC∙- would result in alkylations, and the alkylations that did occur, would be very close to the site of reduction. The net yields of DNA cross-links via this pathway under normoxic conditions would be negligible (Figure 1).
Figure 1:
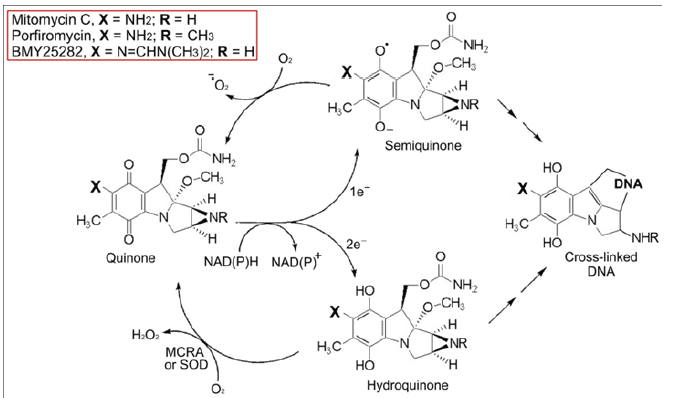
Mitomycin Anoxic Toxicity
Under anoxic and near anoxic conditions, owing to its now extended half-life MC∙- can dismute, or be reduced by low molecular weight cellular reductants to generate mitomycin C hydroquinone (MCH2). The relatively stable two electron reduced product (MCH2) generates highly toxic DNA interstrand cross-linking agents [10,11] (Figure 1). Streptomyces lavendulae produces large quantities of MC yet is exceedingly resistant to its toxic effects [12]. In contrast, a single, MC-induced DNA cross-link in susceptible bacteria results in death [13]. S. lavendulae produces MC Resistance Protein A (MCRA), a 54-kDa flavoprotein [12,14]. The mechanism of resistance by MCRA is via the reoxidation of the two electron reduced product(MCH2) back to the parental drug MC [10,12] (Figure 1). However, this MCRA dependent resistance cannot be expressed under anoxic conditions because the MCRA enzyme requires O2 to function. In simple model systems several peroxidases (horseradish peroxidase; lactoperoxidase; and myeloperoxidase) can perform a similar function to MCRA and protect test DNAs from cross-linking by MCH2 via reoxidation to MC [11]. Very recently Cu/Zn Superoxide Dismutase (SOD) has been shown to possess a non-specific hydroquinone oxidase activity and this likely accounts for its elevated activity in mammalian cells expressing resistance against MC, POR, and related analogues [15]. Therefore, SOD may be regarded as a mammalian MCRA analog (Figure 1). It would be thus expected that tumor targeted inhibition of SOD would likely selectively enhance the anticancer activity of MC type drugs.
The Lethality of DNA Interstrand Cross-Links
The average number of cross-links per DNA molecule (A) can be calculated from the cross-linked fraction, assuming a Poisson distribution such that: A=−ln(1-X), where X=the cross-linked fraction. For a population of test DNA molecules (in this case our test DNA is T7 phage DNA 37,900bp; molecular weight 25×106 Da) with a cross-linked fraction of 0.4, A calculates to be 0.51. The probability that a given DNA molecule has N cross-links equals e-A×AN/N!; where N! equals factorial N. In this case approximately 60% of the DNA molecules would have 0 cross-links, 30.6% would have 1 cross-link, 7.8% would have 2, 1.3% would have 3, etc., to give a total of 40% of the DNA molecules containing one or more cross-links, with the average number of cross-links per DNA molecule (A) being 0.51 [16].This means that to compare the relative cross-linking efficiency of two different cross-linkers (non-nicking/strand breaking cross-linkers, or nearly so) we need to compare the concentrations that produce an equivalent DNA molecule cross-linked fraction (X) or calculate the average number of cross-links per test DNA molecule (A). The importance of DNA interstrand cross-links in the lethality of mitomycin compounds is supported by a study that evaluated the cytotoxicity and DNA cross-links for a series of mitomycin analogs which included, MC, POR, and BMY-25282, as a function of oxygenation [17]. With all of the analogs tested the relationship between the formation of DNA cross-links and the surviving fraction of the treated cells was not significantly different between cells treated under either condition of oxygenation. This suggested that equal number of DNA crosslinks produced an equal cytotoxic effect, indicating that DNA crosslinking was a major lesion contributing to the cytotoxicity for all the mitomycin analogs tested, regardless of the degree of oxygenation.
BMY25282: A Potentially Superior Hypoxia Targeted Anticancer Agent
BMY25282 produced 16% cross-linking at 1.25μM with a vast excess of reducing agent (i.e., all mitomycin reductively activated to the hydroquinone form); and we obtained an equivalent crosslinked fraction by using 40μM MC under the same conditions (methodology described [11]). Thus, BMY25282 requires a ~ 32- fold lower concentration to produce an equivalent yield of DNA cross-links per molecule (Note: This is ~100-fold better than POR). The yield of DNA cross-links (agent potency) has to be high, if you intend to use some vehicle to deliver the agent selectively to the tumor, as the carrying capacity of the vehicle is often limited. It should also be mentioned that in vivo susceptibility to the activated drug would be expected to be higher for a more potent agent such as BMY25282. This is because the required concentration would likely be far lower than the inactivating enzyme’s Km, resulting in less activated agent interception. A therapeutic strategy could be designed, comprising of a copper chelator especially if tumortargeted) coupled with BMY25282 or other mitomycin. In such a strategy, synergy would be expected, as the copper chelator would inhibit tumor detoxification of the activated mitomycin by SOD (Figure 1) [15]. This effect has previously been reported in tissue culture studies [18]. Consistent with the greater DNA crosslinking potency of BMY-25282 reported here in, BMY-25282 was considerably more cytotoxic than MC or POR (based on the IC90 values), under both conditions of oxygenation [17].
Targeting Accuracy and Activity Confinementt
If we assume that a mitomycin activating enzyme acts as a ‘point source’ of activated agent MCact with a half-life of t1/2 it can be shown by applying Fick’s second law of diffusion that the steadystate concentration of activated agent MCact at a distance Δx from the point source of continuous generation can be described by the equation shown below [19].
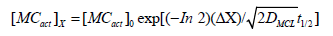
Where, [MCact]X= the concentration of activated mitomycin at distance Δx from the point source; [MCact]0=the concentration of activated mitomycin at the point source of constant generation (the activating enzyme); D=the diffusion coefficient of the activated agent; and t1/2=the half-life of the activated mitomycin. This modeling approach has been previously used to investigate the range of action of the short-lived signaling molecule nitric oxide (NO) from NO generating cells [20], and the distribution of alkylation damage generated by short lived 1,2-bis(sulfonyl)- 1-alkylhydrazines [21]. Diffusion coefficients in free aqueous solutions at 37 °C can be predicted (with reasonable accuracy) using the relationship described by Hobbie RK et al. [22] where the aqueous diffusion coefficient is a function of molecular size (including hydration shell for charged species) and temperature. The extremely short half-life of MC∙- at physiological [O2] would be less than 0.1ms, and this would severely limit the diffusion and alkylation by MC∙- to the immediate proximity of the activating enzyme. This tight activity confinement is clearly illustrated in the observations that the nuclear localization of one electron MC activating enzymes results in increased activity and DNA crosslinks [23,24]. In contrast the much longer half-life of 15s and great permeability of the hydroquinone would extend its toxicity range to an approximate activity radius of 2.5 cell diameters (50μm) [25- 30]. This has the added advantage of providing a bystander effect to enable the kill of tumor cell clones expressing significantly fewer activating enzymes, which may be selected for by using highly focused cytotoxic agents [31-37]. The situation would be far more complex in cells expressing both one and two electron activating enzymes, with various degrees of oxygenation, nevertheless the two extremes described provide an upper and lower limit for the activity ranges of mitomycins and promise favorable utility as therapeutic agents in conjunction with radiation.
References
- Fang J, Nakamura H, Maeda H (2011) The EPR effect: Unique features of tumor blood vessels for drug delivery, factors involved, and limitations and augmentation of the effect. Adv Drug Deliv Rev 63(3): 136-151.
- Tannock IF (1972) Oxygen diffusion and the distribution of cellular radiosensitivity in tumours. Br J Radiol 45: 515-524.
- Wilson WR, Hay MP (2011) Targeting hypoxia in cancer therapy. Nat Rev Cancer 11(6): 393-410.
- Braun RD, Lanzen JL, Snyder SA, Dewhirst MW (2001) Comparison of tumor and normal tissue oxygen tension measurements using oxylite or microelectrodes in rodents. Am J Physiol Heart Circ Physiol 280(6): H2533-2544.
- Brizel DM, Sibley GS, Prosnitz LR, Scher RL, Dewhirst MW (1997) Tumor hypoxia adversely affects the prognosis of carcinoma of the head and neck. Int J Radiat Oncol Biol Phys 38(2): 285-289.
- Rockwell S, Sartorelli AC (1990) Antitumor drug-radiation interactions. In: Hill BT, Bellamy AS (Eds.), (1st edn), CRC Press, Boca Raton, Florida, USA, pp. 125-139.
- Chan DA, Giaccia AJ (2007) Hypoxic, gene expression, and metastasis. Cancer Metastasis Rev 26(2): 333-309.
- Sartorelli AC (1988) Therapeutic attack of hypoxic cells of solid tumors: Presidential address. Cancer Res 48(4): 775-778.
- Kalyanaraman B, Reyes EP, Mason RP (1980) Spin-trapping and direct electron spin resonance investigations of the redox metabolism of quinone anticancer drugs. Biochim Biophys Acta 630(1): 119-130.
- Belcourt MF, Penketh PG, Hodnick WF, Johnson DA, Sherman DH, et al. (1999) Mitomycin resistance in mammalian cells expressing the bacterial mitomycin C resistance protein MCRA. Proc Natl Acad Sci U S A 96(18): 10489-10494.
- Penketh PG, Hodnick WF, Belcourt MF, Shyam K, Sherman DH, et al. (2001) Inhibition of DNA cross-linking by mitomycin C by peroxidase mediated oxidation of mitomycin C hydroquinone. J Biol Chem 276(37): 34445-34452.
- Johnson DA, August PR, Shackleton C, Liu HW, Sherman DH (1997) Microbial resistance to mitomycins involves a redox relay mechanism. J Am Chem Soc 119(10): 2576-2577.
- Hata T, Sano Y, Sugawara R, Matsumae A, Kanamori K, et al. (1956) Mitomycin, a new antibiotic from Streptomyces J Antibiot 9(4): 141-146.
- August PR, Rahn JA, Flickinger MC, Sherman DH (1996) Inducible synthesis of the mitomycin C resistance gene product (MCRA) from Streptomyces lavendulae. Gene 175(2): 261-267.
- Penketh PG (2022) Erythrocuprein, also known as superoxide dismutase, is a hydroquinone oxidase, and imparts resistance to mitomycin C. Reactive Oxygen Species 12: c6-c13.
- Penketh PG, Baumann RP, Ishiguro K, Shyam K, Seow HA, (2008) Lethality to leukemia cell lines of DNA interstrand cross-links generated by cloretazine derived alkylating species. Leuk Res 32(10): 1546-1553.
- Sartorelli AC, Tomasz M, Rockwell S (1993) Studies on the mechanism of cytotoxic action of the mitomycin antibiotics in hypoxic and oxygenated EMT6 cells. Adv Enzyme Regul 33: 3-17.
- Pritsos CA, Keyes SR, Sartorelli AC (1989) Effect of the superoxide dismutase inhibitor, diethyldithiocarbamate, on the cytotoxicity of mitomycin antibiotics. Cancer Biochem Biophys 10(4): 289-298.
- Stanford AL (1975) Foundations of biophysics. (1st edn), Academic Press, New York, USA, pp. 404.
- Lancaster JR (1994) Simulation of the diffusion and reaction of endogenously produced nitric oxide. Proc Natl Acad Sci U S A 91(17): 8137-8141.
- Penketh P, Williamson H, Shyam K (2020) Physicochemical considerations of tumor selective drug delivery and activity confinement with particular reference to 1,2-Bis(sulfonyl)-1- alkylhydrazines delivery. Curr Drug Deliv 17(5): 362-374.
- Hobbie RK, Roth BJ (2007) Intermediate physics for medicine and biology. (4th edn), Springer, Berlin, Germany.
- Holtz KM, Rockwell S, Tomasz M, Sartorelli AC (2003) Nuclear overexpression of NADH: Cytochrome b5 reductase activity increases the cytotoxicity of Mitomycin C (MC) and the total number of MC-DNA adducts in Chinese hamster ovary cells. J Biol Chem 278(7): 5029-5034.
- Seow HA, Belcourt MF, Penketh PG, Hodnick WF, Tomasz M, (2005) Nuclear localization of NADPH: Cytochrome c (P450) reductase enhances the cytotoxicity of mitomycin C to Chinese hamster ovary cells. Mol Pharmacol 67(2): 417-423.
- Penketh PG, Shyam K, Baumann RP, Ratner ES, Sartorelli AC (2015) A simple and inexpensive method to control oxygen concentrations within physiological and neoplastic ranges. Anal Biochem 491: 1-3.
- Pruijn FB, Sturman JR, Liyanage HD, Hicks KO, Hay MP, et al. (2005) Extravascular transport of drugs in tumor tissue: Effect of lipophilicity on diffusion of tirapazamine analogues in multicellular layer cultures. J Med Chem 48(4): 1079-1087.
- Heijden MVD, Jong MCD, Verhagen CVM, Roest RHD, Sanduleanu S, et al. (2019) Acute hypoxia profile is a stronger prognostic factor than chronic hypoxia in advanced stage head and neck cancer patients. Cancers 11(4): 583.
- Lin AJ, Cosby LA, Shansky CW, Sartorelli AC (1972) Potential bioreductive alkylating agents 1 benzoquinone derivatives. J Med Chem 15(12): 1247-1252.
- Lin AJ, Cosby LA, Sartorelli AC (1976) Potential bioreductive alkylating agents. In: Sartorelli AC (Eds.), Cancer Chemotherapy, American Chemical Society, Washington DC, USA, pp. 71-86.
- Lin AJ, Pardini RS, Cosby LA, Lillis BJ, Shansky CW, et al. (1973) Potential bioreductive alkylating agents 2 antitumor effect and biochemical studies of naphthoquinone derivatives. J Med Chem 16(11): 1268-1271.
- Moulder JE, Rockwell S (1987) Tumor hypoxia: Its impact on cancer therapy. Cancer Metastasis Rev 5(4): 313-341.
- Vaupel PW, Frinak S, Bicher HI (1981) Heterogeneous oxygen partial pressure and pH distribution in C3H mouse mammary adenocarcinoma. Cancer Res 41(5): 2008-2013.
- Minchinton AI, Tannock IF (2006) Drug penetration in solid tumours. Nature Rev Cancer 6(8): 583-592.
- Stohrer M, Boucher Y, Stangassinger M, Jain RK (2000) Oncotic pressure in solid tumors is elevated. Cancer Res 60(15): 4251-4255.
- Hicks KO, Pruijn FB, Secomb TW, Hay MP, Hsu R, et al. (2006) Use of three-dimensional tissue cultures to model extravascular transport and predict in vivo activity of hypoxia-targeted anticancer drugs. J Natl Cancer Inst 98(16): 1118-1128.
- Syzbalski W, Iyer VN (1964) Crosslinking of DNA by enzymatically or chemically activated mitomycins and porfirimycins, bifunctional "Alkylating" antibiotics. Fed Proc 23: 946-957.
- Sartorelli AC, Tomasz M, Rockwell S (1993) Studies on the mechanism of the cytotoxic action of the mitomycin antibiotics in hypoxic and oxygenated EMT6 cells. Adv Enzyme Regul 33: 3-17.
© 2023 Philip G Penketh. This is an open access article distributed under the terms of the Creative Commons Attribution License , which permits unrestricted use, distribution, and build upon your work non-commercially.
 a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in
a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in 







.jpg)






























 Editorial Board Registrations
Editorial Board Registrations Submit your Article
Submit your Article Refer a Friend
Refer a Friend Advertise With Us
Advertise With Us
.jpg)






.jpg)














.bmp)
.jpg)
.png)
.jpg)










.jpg)






.png)

.png)



.png)






