- Submissions

Full Text
Modern Applications in Pharmacy & Pharmacology
Efficacy of Transdermal Patches for Elderly Patients with Neurologic Conditions:Preliminary Opinion
Niharika Lal*
Department of Pharmacy, Metro College of Health Sciences and Research, India
*Corresponding author: Niharika Lal, Department of Pharmacy, Metro College of Health Sciences and Research, India
Submission: November 10, 2022;Published: December 15, 2022

ISSN 2637-7756Volume3 Issue1
Abstract
The ease of use, simple and direct treatment plans, avoidance of the first-pass effect, avoidance of high maximum plasma concentrations with rapid changes in drug levels, and lack of invasive procedures associated with intravenous treatment are all advantages that transdermal patches can provide to patients over oral formulations. The mode of drug delivery can be a significant factor in optimising drug therapy as it can affect treatment compliance and outcomes. The development of ideal drug formulations is crucial for treating chronic illnesses or ailments that affect the elderly and for which treatment compliance is known to be low. The characteristics and advantages of transdermal formulations for the treatment of neurological disorders in elderly persons are discussed in this review.
Keywords: Drug; Formulations; Neurological; Disorders; Transdermal patches
Introduction
Due to the increasing prevalence of chronic pain, diabetes mellitus, cardiovascular and neurological illnesses in the aged population, people’s need for drugs rises drastically as they age. Additionally, the elderly needs specific attention paid to drug adherence, drug interactions, and drug delivery. Patients with persistent neurological conditions in particular frequently need numerous drug administrations throughout the day to keep plasma levels steady [1]. Consequently, several attempts have been made to develop pharmacological preparations that can achieve a constant rate of drug delivery. Transdermal lisuride and apomorphine, for instance, have been demonstrated to shorten ‘off’ periods and motor fluctuations in advanced Parkinson’s disease. Additionally, transdermal dopaminergic medications, particularly rotigotine, appear to be the best way to treat people who experience periodic limb movement disorder or restless legs syndrome while they sleep. These conditions are quite common in elderly people or in people who also have neurodegenerative diseases [2].
Because transdermal administration delivers prolonged therapeutic plasma levels of medicines, is easy to use, and may lessen systemic adverse effects, it is the optimal therapeutic method for chronic neurological illnesses in elderly persons. There are now several transdermal delivery systems being researched for the treatment of neuropathic pain, Parkinson’s disease, and Alzheimer’s disease. Even while the majority of transdermal delivery system medications cannot now be regarded as first-line therapies, some of them offer definite advantages over other administration routes and might end up being the preferred option for a subset of patients [3]. To optimize dosages and assess the real incidence of local and systemic side effects, most transdermal therapies still need to be long-term evaluated in large patient groups.
Different methods may improve the pharmacokinetic profile of the medicine or the disease or condition, which can be a crucial factor for prescribing medications. For a number of medications used to treat neurologic diseases, transdermal patch delivery systems have been created, and they may offer pharmacokinetic and practical benefits over oral drug administration. Regarding ease of use and tolerability, drug formulation can also have an impact on a patient’s willingness to undergo a course of therapy [4]. These elements may have a significant impact on treatment adherence and, consequently, treatment results.
The elderly is more likely to have many coexisting disorders and be taking multiple concurrent drugs, so it is crucial to design appropriate therapeutic formulations and modes of therapy for diseases or conditions that primarily impact them. The characteristics and advantages of transdermal formulations for the treatment of neurologic disorders in elderly persons are discussed in this review [5]. Transdermal patch formulations are frequently created to improve drug delivery, effectiveness, and tolerance. They are especially suitable for medications with a short half-life, poor oral absorption, or low oral formulation acceptability. Formulations for transdermal patches offer a non-invasive way to deliver a steady influx of drug molecules into the bloodstream without experiencing the first-pass effect. Although new technological breakthroughs may widen the variety of pharmaceuticals suitable for transdermal delivery, the use of transdermal systems of delivery is limited to those drugs able to penetrate the skin and reach the circulatory system.
Direct drug administration to the circulatory system. Transdermal patches sidestep the digestive system and the hepatic first-pass effect by delivering drugs straight into the circulatory system [6]. This method of distribution is beneficial for the use of Monoamine Oxidase Inhibitors in the treatment of depression (MAOIs). Selegiline inhibits MAO-A as well as MAO-B, while being selective for MAO-B. As a result, the therapeutic usage of oral formulations is constrained by the danger of drug-food interactions brought on by the inhibition of the MAO-A enzyme in the liver and intestine. Due to this inhibition, dietary tyramine may enter the circulatory system and trigger the release of norepinephrine, resulting in a hypertensive emergency. As a result, rigorous dietary restrictions are necessary when using oral MAOIs to reduce tyramine intake [7].
By eliminating the first-pass action in the liver and direct suppression of MAO-A in the gut, the selegiline transdermal system distributes selegiline to the systemic circulation. Without dietary restrictions, the 6-mg/24-hour selegiline patch has been licenced for the treatment of major depressive disorder in the US. Dietary restriction is currently necessary for the 9-mg/24-hour and 12-mg/24-hour doses due to the paucity of safety and tolerability evidence.
In Europe, rotigotine, a transdermal patch-applied dopamine agonist, is now licenced for the treatment of early and advanced Parkinson’s disease as well as restless legs syndrome. Due to a significant first-pass effect and low absorption when taken orally, rotigotine is only available as a transdermal patch formulation. The rotigotine patch, a transdermal formulation, can also provide a non-invasive option for people who are either temporarily or permanently unable to use oral forms [8]. When Parkinson’s disease patients are unable to follow an oral regimen of dopamine agonists during the perioperative period, the patch delivery method, for instance, may be helpful for the management of parkinsonian symptoms.
A small open-label study of Parkinson’s disease patients who required surgery under general anesthesia showed the viability of substituting the rotigotine patch for the patient’s regular treatment regimen. Transdermal patches provide a significant benefit over oral preparations due to their continuous drug delivery, which leads to stable plasma concentrations and lower Cmax values [9]. This consistent and continuous medication delivery can improve the number of patients able to receive therapeutic doses while decreasing tolerability problems brought on by variations in plasma concentrations. Both of these elements are advantageous, particularly for senior people who may be more susceptible to changing plasma levels because of weakened liver and kidney function, additional medical problems, and usage of concurrent drugs [10]. As adverse events and perceived lack of efficacy have both been reported as reasons for noncompliance that are relevant to elderly populations with chronic conditions, the smooth drug delivery of transdermal patches may increase treatment compliance by decreasing adverse events and improving the tolerability of therapeutic doses [11].
Rivastigmine is a cholinesterase inhibitor used to treat dementia caused by Parkinson’s disease and mild to severe Alzheimer’s disease. It comes in oral and transdermal formulations. With the oral rivastigmine capsule formulation, centrally generated cholinergic side effects including nausea and vomiting are linked to both the high Cmax and the quick time from administration to Cmax. The rivastigmine patch was created with the goal of improving patient acceptability because the tolerance of rivastigmine capsules was enhanced when the same daily dosage was provided over 3 doses rather than 2 [12].
For the treatment of Parkinson’s disease patients, transdermal patch formulations of dopamine agonists may be more effective than oral formulations since pulsatile dopaminergic stimulation might cause dyskinesias and other motor problems. Transdermal patches’ continuous and smooth delivery may thereby prevent the emergence of negative effects. The sole dopamine agonist now available in a transdermal formulation, rotigotine, has been found to have clinical efficacy and tolerability comparable to nonergot dopamine agonists taken orally [13]. Table 1 show types of transdermal patches used in neurological disease.
Table 1:Types of transdermal patches.
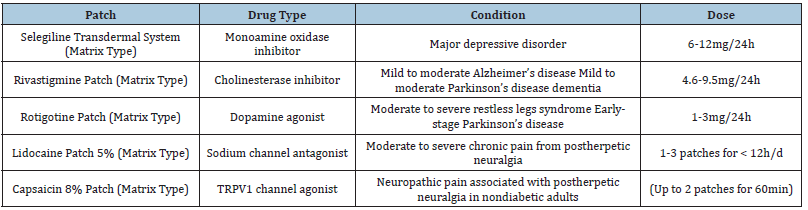
To find out if transdermal distribution lessens the likelihood of motor problems, more research is required. The rotigotine patch has been found to considerably lessen symptoms compared to placebo and to provide maintained efficacy over a two-year period. It is also suggested for the treatment of restless legs syndrome. Continuous medication administration is regarded to be advantageous for people with this disease throughout the day and night. Transdermal patch applications can typically be made without regard to a meal and are easy to use. The physical presence of the patch on the skin is one unique benefit. This presence might give the patient or caregiver visual confirmation that the medication has been given correctly. In a poll of caregivers, choosing transdermal patches over capsules of medicine was frequently cited as a benefit of the patch’s ease of use [14].
Transdermal drugs still have the potential to cause skin application site responses despite recent developments in patch technology. Modern transdermal drugs commonly cause modest application site responses that go away on their own when the patch is removed. The potential benefits of transdermal medication versus oral treatment frequently outweigh any modest skin responses. Patches should be applied to clean, dry, unbroken skin; regular rotation of the application site is essential to prevent cumulative irritating contact dermatitis; and preventative and palliative treatments are required to decrease application site reactions [15].
Conclusion
With regard to convenience of use, straightforward treatment plans, avoiding the first-pass impact, and avoiding peak dosage effects, transdermal patches are a significant medication delivery strategy that can benefit patients over oral formulations. These advantages could be especially important for medication compliance and tolerability in elderly individuals with chronic illnesses like Parkinson’s and Alzheimer’s. Transdermal patches may eventually be used for novel drugs and indications thanks to emerging technologies.
References
- Priano L, Gasco MR, Mauro A (2006) Transdermal treatment options for neurological disorders: Impact on the elderly. Drugs Aging 23(5): 357-375.
- Winblad B, Machado JC (2008) Use of rivastigmine transdermal patch in the treatment of Alzheimer's disease. Expert Opin Drug Deliv 5(12): 1377-1386.
- Grossberg G, Sadowsky C, Förstl H, Frölich L, Nagel J, et al. (2009) Safety and tolerability of the rivastigmine patch: Results of a 28-week open-label extension. Alzheimer Dis Assoc Disord 23(2): 158-164.
- Bunten S, Happe S (2006) Rotigotine transdermal system: A short review. Neuropsychiatr Dis Treat 2(4): 421-426.
- Small G, Dubois B (2007) A review of compliance to treatment in Alzheimer's disease: Potential benefits of a transdermal patch. Curr Med Res Opin 23(11): 2705-2713.
- Kurz A, Farlow M, Lefèvre G (2009) Pharmacokinetics of a novel transdermal rivastigmine patch for the treatment of Alzheimer's disease: A review. Int J Clin Pract 63(5): 799-805.
- Oertel WH, Benes H, Borreguero DG, Högl B, Poewe B, et al. (2010) Rotigotine transdermal patch in moderate to severe idiopathic restless legs syndrome: A randomized, placebo-controlled polysomnographic study. Sleep Med 11(9): 848-856.
- Babbar S, Marier JF, Mouksassi MS, Beliveau M, Vanhove GF, et al. (2009) Pharmacokinetic analysis of capsaicin after topical administration of a high-concentration capsaicin patch to patients with peripheral neuropathic pain. Ther Drug Monit 31(4): 502-510.
- Szunerits S, Boukherroub R (2018) Heat: A highly efficient skin enhancer for transdermal drug delivery. Front Bioeng Biotechnol 6: 15.
- Nair AB, Kumria R, Gupta S, Al-Dhubiab BE (2014) Development and evaluation of a novel drug in adhesive transdermal system of levodopa and carbidopa. J Pharm Innov 9(4): 302-308.
- Akhtar N, Pathak K (2017) Feasibility assessment of transdermal drug delivery systems for treatment of Parkinson’s disease. Annals of Pharmacology and Pharmaceutics 2(17): 1-4.
- Cummings J, Winblad B (2007) A rivastigmine patch for the treatment of Alzheimer’s disease and Parkinson’s disease dementia. Expert Rev Neurother 7(11): 1457-1463.
- Nagy B, Brennan A, Brandtmuller A (2008) The cost-utility of the rivastigmine transdermal patch in the management of patients with moderate Alzheimer’s disease in the US. Poster presented at the American Association for Geriatric Psychiatry Annual Meeting, Orlando, Florida, USA.
- Singh G, Thomas SK, Arcona S, Lingala V, Mithal A (2005) Treatment persistency with rivastigmine and donepezil in a large state medicaid program. J Am Geriatr Soc 53(7): 1269-1270.
- Harada ASM, Vanderplas AM (2006) The effect of adherence to Alzheimer’s disease treatment on healthcare costs in managed care. Poster presented at the International Society for Pharmacoeconomics and Outcomes Research (ISPOR), Philadelphia, USA.
© 2022 Niharika Lal. This is an open access article distributed under the terms of the Creative Commons Attribution License , which permits unrestricted use, distribution, and build upon your work non-commercially.
 a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in
a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in 







.jpg)






























 Editorial Board Registrations
Editorial Board Registrations Submit your Article
Submit your Article Refer a Friend
Refer a Friend Advertise With Us
Advertise With Us
.jpg)






.jpg)














.bmp)
.jpg)
.png)
.jpg)










.jpg)






.png)

.png)



.png)






