- Submissions

Full Text
Modern Applications in Pharmacy & Pharmacology
Review Article: Pharmacology and Analytical Chemistry Profile of Dapagliflozin, Empagliflozin and Saxagliptin
Hany A Batakoushy1, Mahmoud A Omar2,3, Hytham M Ahmed1, Mohamed A Abdel Hamid4 and Mahmoud M Sebaiy5*
1Department of Pharmaceutical Analysis, Egypt
2Department of Pharmacognosy and Pharmaceutical Chemistry, Saudi Arabia
3Department of Analytical Chemistry, Egypt
4Department of pharmaceutical Analytical Chemistry, Egypt
5Department of Medicinal Chemistry, Egypt
*Corresponding author: Mahmoud M Sebaiy, Department of Medicinal Chemistry, Egypt
Submission: February 08 , 2021;Published: March 29, 2022

ISSN 2637-7756Volume2 Issue5
Abstract
Diabetes mellitus is a worldwide disease that requires special and continuous medical care. Many classes of oral hypoglycemic drugs are currently used; however, the treatment strategy depends on the nature of diabetes type, pharmacological properties of the used drugs in addition to the clinical characteristics of the patient. As such, in this literature review, we will shed the light on the pharmacology and analytical chemistry profile of certain oral hypoglycemic drugs specifically Dapagliflozin, Empagliflozin and Saxagliptin that got attention in the last decade. Mode of action and most of up-to-date reported methods that have been developed for determination of these important anti-diabetic drugs in their pure form, combined form with other drugs, combined form with degradation products, and in biological samples are mentioned in detail.
Keywords: Diabetes; Dapagliflozin; Empagliflozin; Saxagliptin; Pharmacology; Analytical chemistr
Introduction
Diabetes mellitus is a lifelong condition requiring continuous medical care. Chronic long-term hyperglycemia associated with diabetes that causes serious complications lead to either drug monitoring in the line of treatment single or combined dosage form. Type 2 Diabetes Mellitus (T2DM) is a worldwide problem affecting approximately 8% of the adult population, with predictions of more than 400 million cases by 2030 [1]. The prevalence of T2DM implies an urgent need for new treatments and preventative strategies. The disease results from progressive β-cell dysfunction in the presence of chronic insulin resistance, leading to a progressive decline in plasma glucose homeostasis, increased glucagon secretion, gluconeogenesis, and renal glucose reabsorption and reduced incretin response. Treatments recommended by the American diabetes association and the European association for the study of diabetes include drugs affecting all of the above processes [2]. Monotherapy with an oral medication should be started concomitantly with intensive lifestyle management. When glycemic control is no longer maintained with a single drug, the addition of a second or third oral hypoglycemic drugs usually more effective than switching to another single drug. Hypoglycemic drugs comprise a chemically and pharmacologically heterogeneous group of drugs. There are different classes of oral hypoglycemic drugs and their selection depends on the nature of diabetes, pharmacological properties of the compounds such as efficacy, safety profile and the clinical characteristics of the patient (stage of disease, age and bodyweight) [3]. These drugs, which exhibit different modes of action may be used as a monotherapy or in various combinations..
Gliflozins
Gliflozins are the newest class of approved oral hypoglycemic agents that specifically inhibit sodium glucose co-transporter 2 function in the kidney, thus preventing renal glucose reabsorption and increasing glycosuria in diabetic individuals while reducing hyperglycemia with a minimal risk of hypoglycemia. They reduce glycated hemoglobin and exert favorable effects beyond glucose control with consistent body weight, blood pressure, and serum uric acid reductions. The main drugs from this group are Dapagliflozin (DGF) and empagliflozin (EGF) [4-8].
Gliptins
Gliptins represent a novel class of agents that improve beta cell health and suppress glucagon, resulting in improved postprandial and fasting hyperglycemia. They function by augmenting the incretin system (GLP-1 and GIP) preventing their metabolism by Dipeptidyl Peptidase-4 (DPP-4). Not only are they efficacious but also safe (weight neutral) and do not cause significant hypoglycemia, making it a unique class of drugs. The main drug from this group is Saxagliptin (SXG) [9].
Mechanism of sodium glucose co-transporter 2 Inhibitors
SGLT2 is a protein in humans that facilitates glucose reabsorption in the kidney. SGLT2 inhibitors block the reabsorption of glucose in the kidney, increase glucose excretion, and lower blood glucose levels. SGLT2 is a low-affinity, high capacity glucose transporter located in the proximal tubule in the kidneys. It is responsible for 90% of glucose reabsorption. Inhibition of SGLT2 leads to decrease in blood glucose due to the increase in renal glucose excretion. The mechanism of action of this new class of drugs also offers further glucose control by allowing increased insulin sensitivity and uptake of glucose in the muscle cells, decreased gluconeogenesis and improved first phase insulin release from the beta cells. Drugs in the SGLT2 inhibitors class include DGF and EGF, these drugs in this class approved by the FDA for the treatment of type 2 diabetes. The usage of studied drugs as tinny amount and very diluted in biological matrix to analyze studied drugs in low levels to be applied in their assay in biological samples and give challenge to find suitable method for analysis of these drugs. Therefore, a new simple and sensitive spectroscopic method was required to achieve the aim of this study. Moreover, it is well-known that spectrofluorimetric methods are much more sensitive than spectrophotometric methods [10]. Furthermore, studied drugs analysis in the required low level in plasma samples by measuring the native fluorescence of each DGF and EGF and needed the use of a fluorogenic derivatizing reagent to enhance the sensitivity of the analysis by producing a highly sensitive fluorophore. Therefore, benzofurazan derivative was used in this study for the first time to develop a new validated and sensitive spectrofluorimetric analytical method for studied drug analysis in all sample matrices either pure or biological. A way to speed up the validation process consists of the use of experimental design, which can be very useful and advantageous for both the evaluation and the optimization of some performance parameters. Experimental design techniques are powerful tools for the exploration of multivariate systems [11- 13]. Statistical design is a way of choosing experiments; efficiently and systematically to give reliable and coherent information. From a statistical standpoint, design means construction of experiments so that the analysis of results yields the maximum amount of information that can be extracted from the experiments. More specifically, experimental design helps the researcher to verify if changes in factor values produce a statistically significant variation of the observed response, and this approach can be used each time it is necessary to have this type of information. Typically, experimental design techniques are used to understand the effect of several variables on a system by a well-defined mathematical model. The strategy is most effective if statistical design is used in most or all stages of development and not only for screening or optimizing the process. A systematic use of statistical design in developing a method ensures traceability, supports validation, and makes the subsequent confirmatory validation much easier and more certain. In fact, it is difficult to completely separate method optimization from validation since these two areas are linked, and sometimes a compromise has to be found [14]. There is no reported voltammetry study for DGF analysis in the literature. DGF acts as electroactive compound and it is easily oxidized. The development of electrochemical-based sensors is considered important. Electrochemical sensors have the reputation of being small, quick, cheap, and easy to use for analytical applications, but their designing to be sensitive and selective for analyte of interest is a challenge. The rapid nature of electrochemistry makes it appealing for use in medical applications where quick tests are necessary for medical diagnostics, to ensure drug quality, and to understand dynamics of molecular changes during diseases. Therefore, polymer films modified electrodes received a great attention recently due to their wide applications in the fields of chemical sensors and biosensors [15-19]. Such modified electrodes can significantly improve the electrocatalytic properties of substrates, decrease the over potential, increase the reaction rate and improve the stability and reproducibility of the electrode response in the area of electro analysis [20-29]. The incorporation of metallic Nanoparticles (NPs) into conductive polymers is of great interest because of their strong electronic interactions between NPs and the polymer matrices. It has been reported that the electrocatalytic properties and conductivities of NPs could be enhanced by the conductive polymeric matrices [19]. Previously, Poly 1,5‑Diaminonaphthalene (PDAN) was prepared in aqueous and nonaqueous media at Glassy Carbon (GC) electrode [20-22]. The electrodeposition of metal NPs in the polymer films improves their tolerance towards electrooxidation of small molecules [30]. Herein, in this perspective, PDAN films were prepared at the surface of GC electrode, followed by monometallic Platinum (Pt) or Palladium (Pd) NPs electrodeposition. Suitability of these new composite NPs modified polymeric GC electrodes towards the electrocatalytic oxidation of studied drugs have been studied by electrochemical measurements. On the other hand, The combination therapy of DGF and SXG was shown to be superior in lowering blood glucose when compared with either of the monotherapy regimens [31]. However, this combination therapy leads to a big challenge in pharmaceutical and biomedical analysis area. Therefore, it is important to get a valid analytical separation technique suitable for the analysis of these drugs in presence of each other. Also, the analysis should be valid in presence of their degradation products and also in pharmaceutical dosage form. High Performance Thin-Layer Chromatography (HPTLC) has several advantages over HPLC in some analysis. As HPTLC, separations are generally more efficient than HPLC. Also, it takes short time for analysis. Moreover, it requires few nanoliter injection volumes. Furthermore, minimal use of solvent and no prior extraction steps compared to HPLC [32,33].
Chemistry of the investigated oral hypoglycemic drugs
The chemical structures and pharmacokinetic parameters of the investigated drugs and their chemical names are presented in Tables 1 & 2.
Table 1:The names, chemical structures and nomenclature of the studied oral hypoglycemic drugs
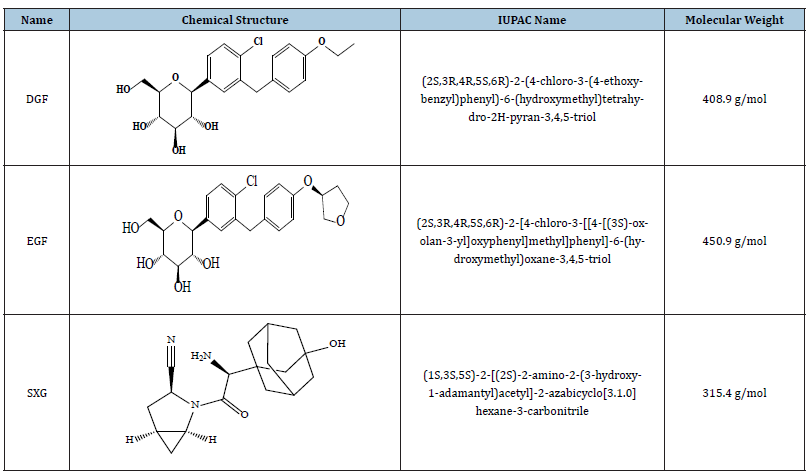
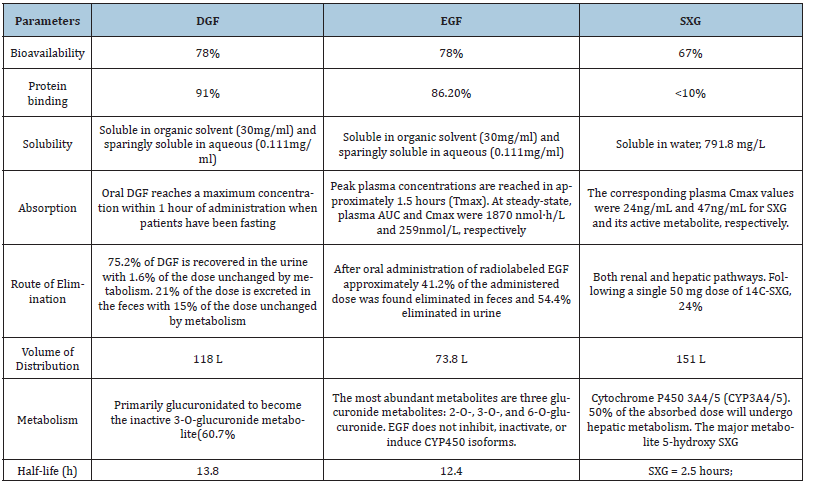
Table 2:Pharmacokinetic and physicochemical parameters of the studied oral antidiabetic drugs.
Analytical methods for the determination of certain antidiabetic drugs
Pharmaceutical analysis has become one of the most important
stages in the therapeutic process. Drug analysis includes analytical
investigations of bulk drug materials, intermediate products, drug
formulations, impurities and degradation products. Analytical
techniques play a significant role in understanding the chemical
stability of the drug, in evaluating the toxicity of some impurities
and in assessing the content of drug in formulations. Also, they
are fundamental tools in pharmacokinetic studies where the
analysis of a drug and its metabolites in biological fluids must be
performed. This review presents analytical procedures such as
spectrophotometric (UV/VIS) methods, HPLC and HPTLC methods.
It is based on a review of the literature from (2009-2020). The
studied drugs (DGF, EGF and SXG) have not an official method in
any pharmacopeia. The reported method included;
i. Spectroscopic methods
ii. Spectrophotometric methods
iii. Ultraviolet and visible spectrophotometric methods:
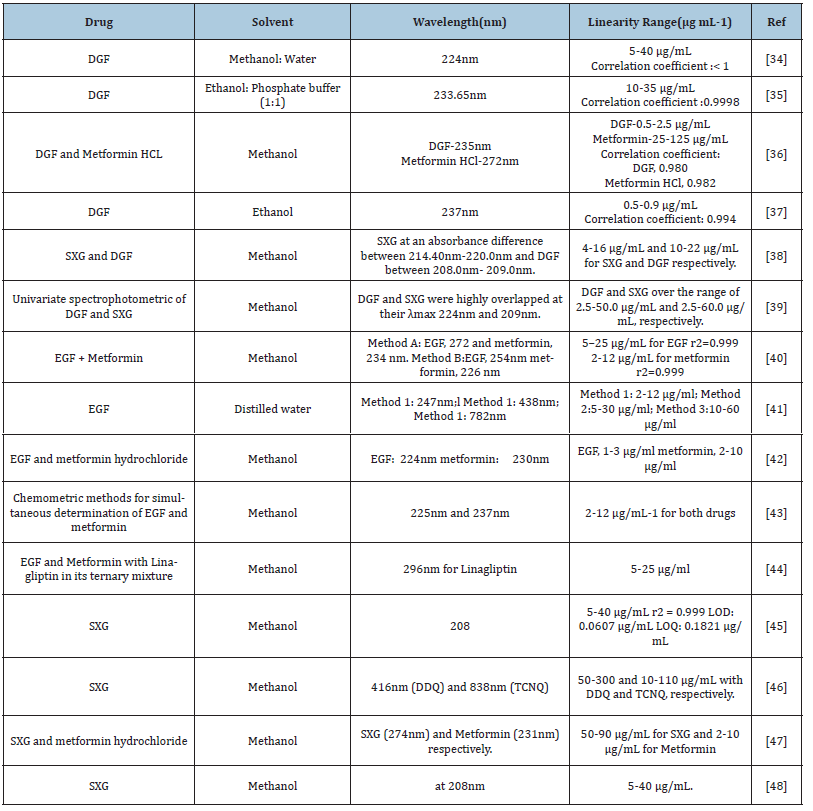
In literature survey, either spectrophotometric methods have been reported for determination of the studied drugs in pure forms or in their pharmaceutical preparations. These reported methods are summarized in Table 3; [34-48].
Table 3:Spectrophotometric (UV/VIS) methods for the analysis of DGF, EGF and SXG in bulk materials and formulations.
Spectrofluorimetric methods
The reported spectrofluorimetric methods for the investigated drugs as the following
Spectrofluorimetric methods of SAX and vildagliptin in bulk and pharmaceutical preparations using NBD-Cl fluorogenic reagent at λex of 468 and 465nm for SAX and VDG, respectively. Fluorescence intensity at λem of 542 and 540nm for SAX and VDG, respectively [49]. A simple and highly sensitive spectrofluorimetric method was developed and validated for the determination of sitagliptin phosphate and SAX. The proposed method is based on Hantzsch reaction of both drugs. Fluorescent products in presence of sodium dodecyl sulfate micellar system as additive to enhance the obtained fluorescence at 483nm after excitation at 419nm for both drugs [50].
High Performance Thin-Layer Chromatography (HPTLC)
A high-performance thin-layer chromatographic method was developed and validated for simultaneous determination of EGF and Linagliptin. The proposed method was applied successfully to the pharmaceutical analysis using precoated silica plates coated with 0.2mm layers of silica gel 60 F254 and methanol: toluene: ethyl acetate (2:4:4, v/v/v) was selected as mobile phase [51]. Stability indicating HPTLC-MS method for estimation of EGF in pharmaceutical dosage form using silica plates coated with 0.2mm layers of silica gel 60 F254 and toluene : methanol (7:3, v/v) was selected as mobile phase [52]. HPTLC was developed for the quantitative analysis of SXG in active pharmaceutical ingredients (APIs) and pharmaceutical dosage forms. The method was achieved using silica gel aluminum plate 60 F254 (10×10cm) as stationary phase and methanol: chloroform (6:4, v/v) as mobile phase [53]. HPTLC method for the simultaneous determination of metformin, SXG and DGF in pharmaceuticals. Separation was performed using aluminum HPTLC sheets coated with silica gel 60 F254 with a mobile phase consisting of a mixture of acetonitrile: 1% w/v ammonium acetate in methanol (9:1,v/v), scanning was performed at 210nm [54]. HPTLC analytical method for simultaneous estimation of DGF and SXG in synthetic mixture using silica gel aluminum plate 60 F254(10×10cm)as stationary phase and chloroform: ethyl acetate: methanol: ammonia (6:2:2:2 drops) as mobile phase [55]. HPTLC method was developed for the determination of either linagliptin, SXG or vildagliptin in their binary mixtures with metformin in pharmaceutical preparations. Separation was carried out on Merck HPTLC aluminum sheets of silica gel 60 F254 using methanol: 0.5% w/v aqueous ammonium sulfate (8:2,v/v) as mobile phase [56].
High Performance Liquid Chromatography (HPLC)
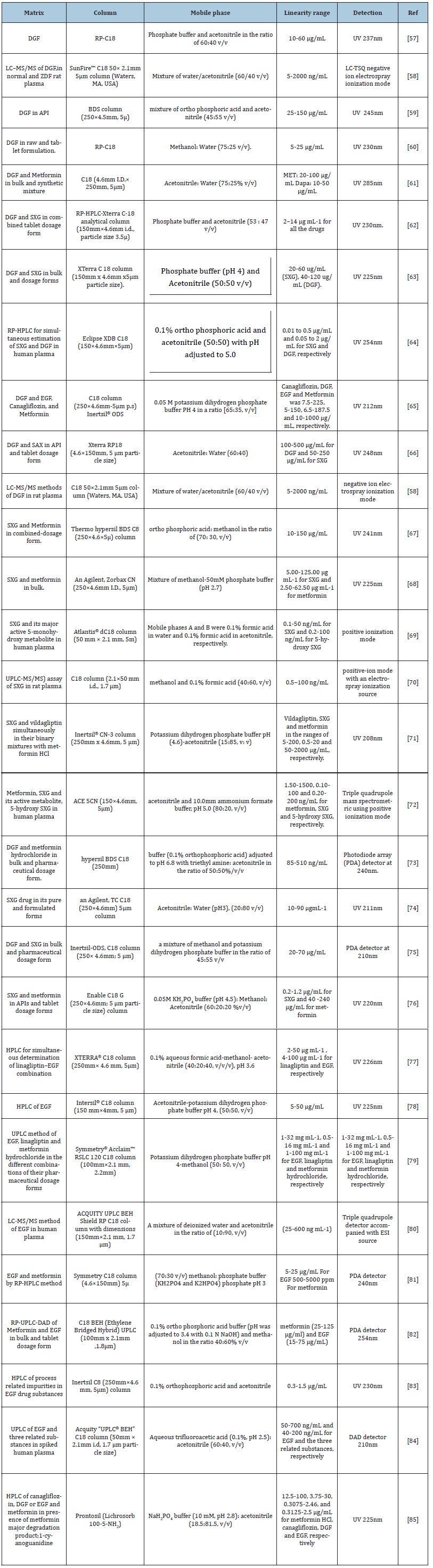
Various HPLC methods had been reported for the determination of the studied drugs either alone or in combination with others active ingredients in dosage forms or in biological fluids. Table 4; [57-85]: summarized the most recent applications of this technique.
Capillary electrophoresis methods
A Capillary Electrophoretic method coupled to a Diode Array Detector (CE-DAD) was developed and validated for the simultaneous determination of metformin hydrochloride, SAX and DGF. The proposed method was used for the determination of these drugs in combinations namely, SXG/metformin, DGF/ metformin and SXG/DGF. CE separation was performed on a fused silica capillary with background electrolyte consisting of 30mM phosphate buffer (pH 6.0). The compounds were detected at 203nm for SXG/DGF and 250nm for metformin. The method was linear in the concentration ranges of 10-200, 1.25-50 and 7.5-100μg/ml for SAX, DGF and metformin, respectively [86].
Table 4:HPLC methods for the analysis of DGF, EGF and SXG in bulk materials and formulations.
Electrochemical method
The literature is devoid of any electrochemical methods for the quantitation of the studied drugs. The first, sensitive and accurate potentiometric sensor for the selective determination of SXG in the presence of either its active metabolite 5‑hydroxy SXG, other coadministered or structurally related drugs [87].
Conclusion
This literature review represents the mode of action in addition to an up-to-date survey about all reported methods that have been developed for determination of Dapagliflozin, Empagliflozin and Saxagliptin in their pure form, combined form with other drugs, combined form with degradation products, and in biological samples such as liquid chromatography, spectrophotometry, spectroflourimetry, electrochemistry, etc.
References
- Zheng Y, Ley SH, Hu FB (2018) Global aetiology and epidemiology of type 2 diabetes mellitus and its complications. Nat Rev Endocrinol 14(2): 88-98.
- Davies MJ, D Alessio DA, Fradkin J, Kernan WN, Mathieu C, et al. (2018) Management of hyperglycaemia in type 2 diabetes, 2018. A consensus report by the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetes Care 41(12): 2669-2701.
- Inzucchi SE, Bergenstal RM, Buse JB, Diamant M, Ferrannini E, et al. (2015) Management of hyperglycaemia in type 2 diabetes, 2015: a patient-centred approach. Update to a position statement of the American Diabetes Association and the European Association for the study of diabetes. Diabetologia 58(3): 429-442.
- Bonner C, Kerr-Conte J, Gmyr V, Queniat G, Moerman E, et al. (2015) Inhibition of the glucose transporter SGLT2 with dapagliflozin in pancreatic alpha cells triggers glucagon secretion. Nat Med 21(5): 512-517.
- Freeman JS (2013) Review of insulin-dependent and insulin-independent agents for treating patients with type 2 diabetes mellitus and potential role for sodium-glucose co-transporter 2 inhibitors. Postgrad Med 125(3): 214-226.
- Grempler R, Thomas L, Eckhardt M, Himmelsbach F, Sauer A, et al. (2012) Empagliflozin, a novel selective Sodium Glucose Co-transporter‐2 (SGLT‐2) inhibitor: characterisation and comparison with other SGLT‐2 inhibitors. Diabetes Obes Metab 14(1): 83-90.
- Neumiller JJ (2014) Empagliflozin: a new sodium-glucose co-transporter 2 (SGLT2) inhibitor for the treatment of type 2 diabetes. Drugs Context 3: 212262.
- Washburn WN, Poucher SM (2013) Differentiating sodium-glucose co-transporter-2 inhibitors in development for the treatment of type 2 diabetes mellitus. Expert Opin Investig Drugs 22(4): 463-486.
- Scirica BM, Braunwald E, Raz I, Cavender MA, Morrow DA, et al. (2014) Heart failure, saxagliptin, and diabetes mellitus: observations from the SAVOR-TIMI 53 randomized trial. Circulation 130(18): 1579-1588.
- Derayea SM, Hamad AA, Nagy DM, Nour-Eldeen DA, Ali HRH (2018) Improved spectrofluorimetric determination of mebendazole, a benzimidazole anthelmintic drug, through complex formation with lanthanum (III); Application to pharmaceutical preparations and human plasma. Journal of Molecular Liquids 272: 337-343.
- Carlson R, Carlson JE (2005) Design and optimization in organic synthesis. (1st edn), Elsevier, Netherlands.
- Lewis G, Mathieu D, Phan TLR (1999) Pharmaceutical Experimental Design. Mixtures in a constrained region of interest. Dekker, USA, pp. 413-454.
- Montgomery DC (2001) Design and analysis of experiments. John Wiley & Sons Inc, (8th edn), USA.
- Furlanetto S, Orlandini S, Mura P, Sergent M, Pinzauti S (2003) How experimental design can improve the validation process. Studies in pharmaceutical analysis. Anal Bioanal Chem 377(5): 937-944.
- Ensafi AA, Rezaei B, Amini M, Heydari Bafrooei E (2012) A novel sensitive DNA–biosensor for detection of a carcinogen, Sudan II, using electrochemically treated pencil graphite electrode by voltammetric methods. Talanta 88: 244-251.
- Gong W, Dou ZY, Liu P, Cai XY, He XQ (2012) Simultaneous determination of dopamine, ascorbic acid by Polyethylene Oxide (PEO) covalently modified glassy carbon electrode. Journal of Electroanalytical Chemistry 666: 62-66.
- Hadi M, Rouhollahi A (2012) Simultaneous electrochemical sensing of ascorbic acid, dopamine and uric acid at anodized nanocrystalline graphite-like pyrolytic carbon film electrode. Anal Chim Acta 721: 55-60.
- Yang L, Liu S, Zhang Q, Li F (2012) Simultaneous electrochemical determination of dopamine and ascorbic acid using AuNPs@ polyaniline core–shell nanocomposites modified electrode. Talanta 89: 136-141.
- Yuan Y, Li H, Han S, Hu L, Parveen S (2012) Immobilization of tris (1, 10-phenanthroline) ruthenium with graphene oxide for electrochemiluminescent analysis. Anal Chim Acta 720: 38-42.
- Abdel-Azzem M, Yousef U, Pierre G (1998) A cyclic voltammetric and coulometric study of a modified electrode prepared by electrooxidative polymerization of 1, 5-diaminonaphthalene in aqueous acidic medium. European Polymer Journal 72(5-6): 819-826.
- Adzic RR, Hsiao MW, Yeager EB (1989) Electrochemical oxidation of glucose on single crystal gold surfaces. Electrochem Soc 143(3): 475-485.
- Azzem MA, Yousef U, Limosin D, Pierre G (1994) Electropolymerization of 1, 5-diaminonaphthalene in acetonitrile and in aqueous solution. Synthetic Metals 63(1): 79-81.
- Bai Y, Yang W, Sun Y, Sun C (2008) Enzyme-free glucose sensor based on a three-dimensional gold film electrode. Sensors Actuators. B 134(2): 471-476.
- Barrera C, Zhukov I, Villagra E, Bedioui F, Aez MAP et al. (2006) Journal of Electroanalytical Chemistry 589: 212.
- Jafarian M, Forouzandeh F, Danaee I, Gobal F, Mahjani M (2009) Electrocatalytic oxidation of glucose on Ni and NiCu alloy modified glassy carbon electrode. Journal of Solid State Electrochemistry 13: 1171-1179.
- Kalimuthu P, John SA (2010) Simultaneous determination of ascorbic acid, dopamine, uric acid and xanthine using a nanostructured polymer film modified electrode. Talanta 80(5): 1686-1691.
- Tominaga M, Shimazoe T, Nagashima M, Kusuda H, Kubo A (2006) Electrocatalytic oxidation of glucose at gold–silver alloy, silver and gold nanoparticles in an alkaline solution. Journal of Electroanalytical Chemistry 590(1): 37-46.
- Wang CH, Yang C, Song YY, Gao W, Xia HX (2005) Adsorption and direct electron transfer from hemoglobin into a three‐dimensionally ordered macroporous gold film. Advanced Functional Materials 15(8): 1267-1275.
- Wang HS, Li TH, Jia WL, Xu HY (2006) Highly selective and sensitive determination of dopamine using a Nafion/carbon nanotubes coated poly (3-methylthiophene) modified electrode. Biosens Bioelectron 22(5): 664-669.
- Pournaghi-Azar MH, Habibi B (2007) Electrocatalytic oxidation of methanol on poly (phenylenediamines) film palladized aluminum electrodes, modified by Pt micro-particles: comparison of permselectivity of the films for methanol. Journal of Electroanalytical Chemistry, p-10.
- Singh-Franco D (2015) Potential for dipeptidyl peptidase-4 inhibitor and sodium glucose cotransporter 2 inhibitor single-pill combinations. Expert Rev Endocrin Metab 10(3): 305-317.
- Mohamed AMI, Omar MA, Derayea SM, Hammad MA, Mohamed AA (2018) Innovative thin-layer chromatographic method combined with fluorescence detection for specific determination of Febuxostat: Application in biological fluids. Talanta 176: 318-328.
- Saraya RE, Elhenawee M, Saleh H (2018) Development of a highly sensitive high‐performance thin‐layer chromatography method for the screening and simultaneous determination of sofosbuvir, daclatasvir, and ledipasvir in their pure forms and their different pharmaceutical formulations. J Sep Sci 41(18): 3553-3560.
- Mante GV, Gupta KR, Hemke AT (2017) Estimation of Dapagliflozin from its tablet formulation by UV-Spectrophotometry. Pharm Methods 8(2): 102-107.
- Karuna PC, China EM, Rao MB (2015) Unique UV spectrophotometric method for reckoning of dapagliflozin in bulk and pharmaceutical dosage forms. J Chem Pharm Res 7(9): 45-49.
- Jani BR, Shah KV, Kapupara PP (2015) Development and Validation of UV Spectroscopic First Derivative Method for Simultaneous Estimation of Dapagliflozin and Metformin Hydrochloride in Synthetic Mixture. J Bioequiv 1(1): 102.
- Manasa S, Dhanalakshmi K, Nagarjuna R, Sreenivasa S (2014) Method development and validation of dapagliflozin in API by RP-HPLC and UV-spectroscopy. IJPSDR 6(3): 250-252.
- Suthar AM, Prajapati LM, Joshi AK, Patel JR, Kharodiya ML (2018) Estimation of Saxagliptin hydrochloride and Dapagliflozin propendiol monohydrate in combined dosage form. JIAPS 3(2): 01-07.
- Lotfy HM, Mohamed D, Elshahed MS (2019) Novel univariate spectrophotometric determination of the recently released solid dosage form comprising dapagliflozin and saxagliptin via factorized response spectra: Assessment of the average content and dosage unit uniformity of tablets. Spectrochimica Acta Part A: Molecular and Biomolecular Spectroscopy 222.
- Padmaja N, Babu MS, Veerabhadram G (2016) Development and validation of UV spectrophotometric method for simultaneous estimation of smpagliflozin and metformin hydrochloride in bulk drugs and combined dosage forms. Der Pharmacia Lettre 8(13): 207-213.
- Jyothirmai N, Nagaraju B, Anil Kumar M (2016) Novel uv and visible spectrophotometric methods for the analysis of empagliflozin a type 2 diabetic drug in bulk and pharmaceutical formulations. Journal De Afrikana 3(1): 177-187.
- Patil SD, Chaure SK, Kshirsagar S (2017) Development and validation of UV spectrophotometric method for Simultaneous estimation of Empagliflozin and Metformin hydrochloride in bulk drugs. AJPAna 7(2): 117-123.
- Ayoub BM (2016) Development and validation of simple spectrophotometric and chemometric methods for simultaneous determination of empagliflozin and metformin: Applied to recently approved pharmaceutical formulation. Spectrochim Acta A Mol Biomol Spectrosc 168: 118-122.
- Shaker L (2016) Development of Economic UV Spectrophotometric method for determination of linagliptin in its tertiary mixture with empagliflozin and metformin: comparision to economic pharmaceutical analysis literature. Scholars Research Library 8(13): 267-269.
- Kalaichelvi R, Jayachandran E (2011) Validated spectroscopic method for estimation of saxagliptin in pure and from tablet formulation. Int J Pharm Pharm Sci 3(3): 179-180.
- El-Bagary RI, Elkady EF, Ayoub BM (2012) Spectrophotometric methods based on charge transfer complexation reactions for the determination of saxagliptin in bulk and pharmaceutical preparation. Int J Biomed Sci 8(3): 204-208.
- Narendra N, Govinda J (2012) Development and validation of uv-vis spectroscopy method for simultaneous estimation of saxagliptin hydrochloride and metformin hydrochloride in active pharmaceutical ingredient. J Pharm Educ Res 3(2): 19-23.
- Kalaichelvi R, Jayachandran E (2011) Validated Spectroscopic method for the estimation of Saxagliptinin pure and from tablet formulation. Int J Pharm Pharm Sci 3(3): 179-180.
- Moneeb MS (2013) Spectrophotometric and spectrofluorimetric methods for the determination of saxagliptin and vildagliptin in bulk and pharmaceutical preparations. Bulletin of Faculty of Pharmacy, Cairo University 51(2): 139-150.
- Barseem A, Ahmed H, El-Shabrawy Y, Belal F (2019) The use of SDS micelles as additive to increase fluorescence analysis of sitagliptin and saxagliptin derivatives in their tablets and human plasma. Microchemical Journal 146: 20-24.
- Bhole R, Wankhede S, pandey M (2017) Stability indicating HPTLC method for simultaneous estimation of empagliflozin and linagliptin in pharmaceutical formulation. Analytical Chemistry Letters 7(1): 76-85.
- Bhole RP, Tamboli FR (2018) Development and validation of stability indicating HPTLC-MS method for estimation of empagliflozin in pharmaceutical dosage form. Analytical Chemistry Letters 8(2): 244-256.
- Srividya S, Swetha E, Veeresham C (2015) Development and validation of a high performance thin layer chromatographic method for quantitative analysis of saxagliptin. American journal of analytical chemistry 6(10): 797-806.
- Afnan EA, Hadir MM, Nourah ZA (2020) HPTLC Method for the determination of metformin hydrochloride, saxagliptin hydrochloride and dapagliflozin in pharmaceuticals. Current Analytical Chemistry 16(5): 609-619.
- Shveta HP, Shailesh VL, Sachin BN (2018) Development and validation of UV-spectroscopic first derivative and high performance thin layer chromatography analytical methods for simultaneous estimation of dapagliflozin propanediol monohydrate and saxagliptin hydrochloride in synthetic mixture. EJBPS 5(5): 668-681.
- El-Kimary EI, Hamdy DA, Mourad SS, Barary MA (2016) HPTLC determination of three gliptins in binary mixtures with metformin. J Chromatogr Sci 54(1): 79-87.
- Debata J, Kumar S, Jha SK, Khan A (2017) A new RP-HPLC method development and validation of dapagliflozin in bulk and tablet dosage form. Int J Drug Dev & Res 9(2): 48-51.
- Aubry AF, Gu H, Magnier R, Morgan L, Xu X, et al. (2010) Validated LC-MS/MS methods for the determination of dapagliflozin, a sodium-glucose co-transporter 2 inhibitor in normal and ZDF rat plasma. Bioanalysis 2(12): 2001-2009.
- Manasa S, Dhanalakshmi K, Nagarjunareddy G, Sreenivasa S (2014) Development and validation of a RP-HPLC method for the estimation of dapagliflozin in API. International Journal of Pharmaceutical Sciences and Research 5(12): 5394-5397.
- Manoharan G, Ismaiel AM, Ahmed ZM (2018) Stabilityindicating RP-HPLC method development for simultaneous determination & estimation of dapagliflozin in raw & tablet formulation. Chemistry Research Journal 3(2): 159-164.
- Urooj A, Sundar PS, Vasanthi R, Raja MA, Dutt KR, et al. (2017) Development and validation of RP-HPLC method for simultaneous estimation of dapagliflozin and metformin in bulk and in synthetic mixture. World journal of pharmacy and pharmaceutical sciences 6(7): 2139-2150.
- Singh N, Bansal P, Maithani M, Chauhan Y (2018) Development and validation of a stability-indicating RP-HPLC method for simultaneous determination of dapagliflozin and saxagliptin in fixed-dose combination. New Journal of Chemistry 42(4): 2459-2466.
- Kommineni V, Chowdary K, Prasad S (2017) Development of a new stability indicating RP-HPLC method for simultaneous estimation of saxagliptine and dapagliflozin and its validation as per ich guidelines. IAJPS 4(9): 2920-2932.
- Sharmila D, Suneetha A (2019) Simultaneous estimation of saxagliptin and dapagliflozin in human plasma by validated high performance liquid chromatography-ultraviolet method. Turk J Pharm Sci 16(2): 227-233.
- Khalil GA, Salama I, Gomaa MS, Helal MA (2018) Validated RP-HPLC method for simultaneous determination of canagliflozin, dapagliflozin, empagliflozin and metformin. IJPCBS 8(1): 1-13.
- Deepan T, Dhanaraju MD (2018) Stability indicating HPLC method for the simultaneous determination of dapagliflozin and saxagliptin in bulk and tablet dosage form. Current Issues in Pharmacy and Medical Sciences 31(1): 39-43.
- Bhagavanji N (2012) Development and validation of stability indicating LC method for the simultaneous estimation of metformin and saxagliptin in combined dosage form. VSRD International Journal of Technical & Non-Technical Research 3(11): 1-19.
- Caglar S, Ali RA (2014) A validated high performance liquid chromatography method for the determination of saxagliptin and metformin in bulk, a stability indicating study. J Anal Bioanal Techs 12: 2.
- Demers R, Gu H, Christopher LJ, Su H, Cojocaru L, et al. (2012) Liquid chromatography and tandem mass spectrometry method for the quantitative determination of saxagliptin and its major pharmacologically active 5-monohydroxy metabolite in human plasma: method validation and overcoming specific and non-specific binding at low concentrations. J Chromatogr B Analyt Technol Biomed Life Sci 889-890: 77-86.
- Gao JW, Yuan YM, Lu YS, Yao MC (2012) Development of a rapid UPLC‐MS/MS method for quantification of saxagliptin in rat plasma and application to pharmacokinetic study. Biomed Chromatogr 26(12): 1482-1487.
- Mohammad MAA, Elkady EF, Fouad MA (2012) Development and validation of a reversed-phase column liquid chromatographic method for simultaneous determination of two novel gliptins in their binary mixtures with Metformin. European Journal of Chemistry 3(2): 152-155.
- Shah PA, JV Shah, M Sanyal, PS Shrivastav (2017) LC–MS/MS analysis of metformin, saxagliptin and 5‐hydroxy saxagliptin in human plasma and its pharmacokinetic study with a fixed‐dose formulation in healthy Indian subjects. Biomed Chromatogr 31(3).
- Yunoos M, Sankar DG (2015) A validated stability indicating high-performance liquid chromatographic method for simultaneous determination of metformin Hcl and dapagliflozin in bulk drug and tablet dosage form. Asian J Pharm Clin Res 8(3): 320-326.
- Daswadkar SC, Roy MA, Walode SG, Mahendra KC (2016) Quality by design approach for the development and validation of saxagliptin by RP-HPLC with application to formulated forms. Int J Pharm Sci 7(4): 1670-1677.
- Aswini R, Eswarudu MM, Srinivasa BP (2018) A review on analytical methods for estimation of dapagliflozin and saxagliptin in bulk and in pharmaceutcal dosage forms. IJRPC 8(3): 460-468
- Prasad PBN, Satyanaryana K, Krishnamohan G (2015) Development and Validation of a Method for Simultaneous Determination of Metformin and Saxagliptin in a Formulation by RP-HPLC. American Journal of Analytical Chemistry 6(11): 841-850.
- Abdel-Ghany MF, Abdel-Aziz O, Ayad MF, Tadros MM (2017) New LC–UV methods for pharmaceutical analysis of novel anti-diabetic combinations. Acta Chromatographica 29(4): 448-452.
- Abdel‐Ghany MF, Ayad MF, Tadros MM (2018) Liquid chromatographic and spectrofluorimetric assays of empagliflozin: Applied to degradation kinetic study and content uniformity testing. Luminescence 33(5): 919-932.
- Ayoub BM (2015) UPLC simultaneous determination of empagliflozin, linagliptin and metformin. RSC advances 5(116): 95703-95709.
- Ayoub BM, Mowaka S, Elzanfaly ES, Ashoush N, Elmazar MM, et al. (2017) Pharmacokinetic evaluation of empagliflozin in healthy egyptian volunteers using lc-ms/ms and comparison with other ethnic populations. Scientific reports 7(1): 2583.
- Godasu S, Sreenivas S (2017) A new validated RP-HPLC method for the determination of Metformin HCL and Empagliflozin in its bulk and pharmaceutical dosage forms. IJPSR 8(5): 2223-2232.
- Padmaja N, Veerabhadram G (2017) A novel stability indicating rp-uplc-dad method for determination of metformin and empagliflozin in bulk and tablet dosage form. Oriental Journal of Chemistry 33(4): 1949-1958.
- Jaiswal SH, Katariya M, Katariya V, Karva G, Koshe K (2017) Validated stability indicating hplc method for determination of process related impurities in empagliflozin drug substances. World Journal of Pharmaceutical Research 6: 8741.
- Mabrouk MM, Soliman SM, El-Agizy HM, Mansour FR (2019) A UPLC/DAD method for simultaneous determination of empagliflozin and three related substances in spiked human plasma. BMC Chemistry 13(1): 83.
- Hassib ST, Taha EA, Elkady EF, Barakat GH (2019) Validated liquid chromatographic method for the determination of (canagliflozin, dapagliflozin or empagliflozin) and metformin in the presence of (1-cyanoguanidine). J Chromatogr Sci 57(8): 697-707.
- Maher HM, Abdelrahman AE, Alzoman NZ, Aljohar HI (2019) Stability-indicating capillary electrophoresis method for the simultaneous determination of metformin hydrochloride, saxagliptin hydrochloride, and dapagliflozin in pharmaceutical tablets. Journal of Liquid Chromatography & Related Technologies 42(5-6): 161-171.
- Abdallah N, Ibrahim HF (2019) Electrochemical determination of Saxagliptin hydrochloride with MWCNTs/CuO/4′ aminobenzo-18-crown-6-ether composite modified carbon paste electrode. Microchemical Journal 147: 487-496.
© 2022 Mahmoud M Sebaiy. This is an open access article distributed under the terms of the Creative Commons Attribution License , which permits unrestricted use, distribution, and build upon your work non-commercially.
 a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in
a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in 







.jpg)






























 Editorial Board Registrations
Editorial Board Registrations Submit your Article
Submit your Article Refer a Friend
Refer a Friend Advertise With Us
Advertise With Us
.jpg)






.jpg)














.bmp)
.jpg)
.png)
.jpg)










.jpg)






.png)

.png)



.png)






