- Submissions

Full Text
Modern Applications in Pharmacy & Pharmacology
Evaluation of Antioxidant Activity and Free Radical Scavenging Potential of the Biofield Energy Healing Treated Novel Proprietary Formulation
Mahendra Kumar Trivedi1 and Snehasis Jana2*
1Trivedi Global, Inc., USA
2Trivedi Science Research Laboratory Pvt. Ltd., Thane (W), Maharashtra, India
*Corresponding author: Snehasis Jana, Trivedi Science Research Laboratory, India
Submission: April 16, 2018;Published: August 12, 2019

ISSN 2637-7756Volume2 Issue4
Abstract
The present study was aimed to evaluate the antioxidant and free-radical scavenging potential of the Biofield Energy Healing (The Trivedi Effect®) Treated novel proprietary test formulation in human liver cancer (HepG2) cells. The test formulation was divided into two parts. One part was denoted as the control, which did not receive the Biofield Energy Treatment, while the other part received the Biofield Treatment, and defined as the Biofield Energy Treated test formulation. The test formulation was evaluated for antioxidant enzymes activities such as superoxide dismutase (SOD), catalase (CAT), lipid peroxidation (LPO), cell viability against oxidative damage, and 2,2-azino-bis (3-ethylbenzothiazoline-6-sulphonic acid) (ABTS) analysis. 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay data showed that the test formulation was noticed as a safe and non-toxic profile. The experimental results showed that the SOD level was significantly increased by 26.57% and 45.55% at 0.0065 and 0.0033μg/ ml, respectively as compared with the untreated test formulation. Further, CAT enzyme activity was improved by 28.57% at 1.04μg/ml as compared with the untreated test formulation. Similarly, LPO activity was significantly decreased by 26.46%, 29.02%, and 32.16% at 10.41, 0.104, and 0.05μg/ml, respectively with respect to the untreated test formulation. Free-radical scavenging activity using ABTS assay was inhibited by 9.8% at 1% with respect to the untreated test formulation. Overall, data suggested that the Biofield Energy Treated test formulation has significantly improved the antioxidant functions and could be useful against many autoimmune and inflammatory diseases, stress management and prevention. It can also be used as anti-aging therapy and for the improvement of body’s detoxification process.
Keywords: Consciousness energy healing; The Trivedi effect®; Free-radical; Anti-oxidation; Oxidative stress; HepG2 cells
Introduction
Oxygen is a crucial element for life that can produce highly reactive compounds known as reactive oxygen species (ROS) as well as reactive nitrogen species (RNS) such as free radicals containing an unpaired electron, which might cause serious deleterious toxic effects on the body. These free radicals can attack several macromolecules such as lipids, proteins, and DNA causing cellular damage. Oxidative stress results between the imbalance among production of ROS and the defense systems to readily detoxify them [1]. Thus, at low or moderate levels, these reactive compounds exert valuable effects on the cellular responses and immune function. However, at high concentrations, a deleterious process known as oxidative stress occurred which damage all cell structures [2]. Most of the chronic and degenerative ailments are directly or indirectly related with the oxidative stress such as cancer, arthritis, aging, autoimmune disorders, cardiovascular and neurodegenerative diseases [3]. However, the human body has numerous mechanisms to neutralize the generated oxidative stress by generating different antioxidants that are either naturally produced in situ, or externally provided via foods and/ or supplements. Endogenous and exogenous antioxidants worked as free radical scavengers, they prevent and repair the damaged caused by ROS and RNS, which results in improved immune defense and reduced the risk of cancer and degenerative diseases [4]. Complementary and Alternative Medicines (CAM) are the best source of exogenous antioxidants, which has become increasingly popular in the developed world [5,6]. Plants or plant-based products are preferred as the key source of treatment strategy in various medicinal systems. In recent years, a combination of herbal product (polyherbal) or single herbs has been used as a curative substance in order to improve the health conditions. Evidence-based medicines are accepted worldwide and National Center for Complementary and Alternative Medicine (NCCAM) has been inaugurated as the United States Federal Government’s lead agency for conducting scientific research and practicing in the arena of medicine [7]. Thus, these herbomineral combinations as nutritional supplements are preferred as a best choice due to the presence of minerals and vitamins, however lack of nutritional supplements results in infectious and immunological diseases results in morbidity and mortality [8]. Most of the synthetic nutritional supplements are associated with adverse effects [9]. Thus, one novel test formulation was designed based on nanocurcumin, zinc chloride, magnesium (II) gluconate hydrate, sodium selenate, ascorbic acid (vitamin C), cholecalciferol (vitamin D3), iron (II) sulfate, and copper chloride. All the active constituents of the novel formulation such as nanocurcumin, minerals, and vitamins were reported to have significant antioxidant and immunological activities [10-12].
Biofield Energy Healing Treatment as a CAM approach has been reported to have significant outcomes against various disease conditions. National Institute of Health (NIH) recommend and included various Energy therapies such as natural products, deep breathing, yoga, chiropractic/osteopathic manipulation, meditation, massage, special diets, homeopathy, progressive relaxation, guided imagery, acupressure, acupuncture, relaxation techniques, hypnotherapy, healing touch, movement therapy, pilates, rolfing structural integration, mindfulness, Ayurvedic medicine, traditional Chinese herbs and medicines, naturopathy, essential oils, aromatherapy, Reiki, cranial sacral therapy and applied prayer (as is common in all religions, like Christianity, Hinduism, Buddhism and Judaism) under CAM category that has been accepted by the most of the U.S. population with several advantages [13]. Every living organism possess some unique energy that can be harness and transmit it into other living and non-living things by the process of Biofield Energy Healing by altered atomic/molecular weights through possible mediation of neutrinos [14]. Biofield Energy Healing Treatment (The Trivedi Effect®- Consciousness Energy Healing) have been studied and reported with significant outcomes in various scientific disciplines such as microbiology with altered antimicrobial sensitivity against pathogenic microbes [15-17], genetics [18-20], skin health [21,22], agricultural science [23,24], immunity [25,26], pharmaceuticals [27,28], and materials science [29,30]. In the present study, the authors evaluated the impact of the Biofield Energy (The Trivedi Effect®-Consciousness Energy Healing) Treatment on the novel test formulation for its anti-oxidation action using standard assays.
Materials and Methods
Chemicals and reagents
3-(4, 5-dimethyl-2-thiazolyl) 2, 5 diphenyl-2 H-tetrazolium) (MTT), trypsin, EDTA, iron sulfate, copper chloride, and cholecalciferol (vitamin D3 ) and FBS were procured from SigmaAldrich (St. Louis, MO, USA). Antibiotics solution (PenicillinStreptomycin) and EMEM were purchased from HiMedia, India. ABTS [2,2-zinobis-(3-ethylbenzothiazoline-6-sulfonic acid)] was purchased from Amresco, USA, while DMSO was purchased from Fischer Scientific. Nanocurcumin was purchased from Sanat Products Ltd., India. Zinc chloride and magnesium (II) gluconate hydrate were obtained from TCI, Japan. Sodium selenate and ascorbic acid were procured from Alfa Aesar, USA. All other chemicals used in this study were analytical grade available in India.
Test formulation and reference standard
The novel test formulation contained a combination of nanocurcumin along with minerals viz. iron sulfate, copper chloride, zinc chloride and magnesium (II) gluconate hydrate, vitamins viz. cholecalciferol (vitamin D3 ) and ascorbic acid (Vitamin C). Quercetin (20mM stock solution of quercetin was prepared in DMSO) or ascorbic acid (dissolved in 80% methanol to obtain the stock solution of 1mg/mL) used as a reference standard (positive control) for anti-oxidation action in HepG2 cells.
Biofield energy healing strategies
One part of the novel proprietary test formulation did not receive any sort of treatment and was defined as the untreated test formulation, while another part received Biofield Energy Treatment under standard laboratory conditions, known as the Biofield Treated Test formulation. The Biofield Energy Treatment on test formulation was administered by Mahendra Kumar Trivedi [14- 30], a renowned Biofield Energy Healer (The Trivedi Effect®) under similar conditions for 5 minutes. This treatment was provided through the Biofield Energy Healer unique Energy Transmission process (The Trivedi Effect®) to the test formulation. The Biofield Energy Healer was located in the USA, however the test items were located in the research laboratory of Dabur Research Foundation, New Delhi, India. Biofield Energy Healer in this experiment did not visit the laboratory, nor had any contact with the test samples. Further, the control group was treated by a sham healer for comparison purpose. The “sham” healer did not aware about the Biofield Energy Treatment. After that, the Biofield Energy treated and untreated samples were kept for the in vitro antioxidant study.
Experimental design
HepG2 cells were divided in 5 experimental groups. Group 1 comprised of the HepG2 cells and denoted as the untreated cells, group 2 included cells in presence of H2O2 (20mM) as a negative control group with oxidative stress. Group 3 included Quercetin or ascorbic acid with different concentrations as a positive control group, group 4 included HepG2 cells in the presence of untreated novel test formulation and group 5 denoted as Biofield Energy Treated test formulation at various concentrations. After 24 hours of incubation, supernatants were analyzed for absorbance in different anti-oxidant parameters, which were read using Synergy HT microplate reader as per the manufacturer’s instructions. Concentrations were measured as triplicate wells of every sample.
Cell culture and test formulation treatment
HepG2 cells (Hepatocellular carcinoma) were used for antioxidant activity, which was purchased from National Centre for Cell Science, India. Cells were grown in 96-well culture plates by EMEM medium supplemented with 100units/ml of penicillin, 10% FBS, and 100μg/ml of streptomycin. Sub-culturing of cells was performed in trypsin (0.2%) and EDTA (0.02%) by trypsinization followed by splitting the cell suspension into new flasks and supplementing with fresh cell growth medium. Further, the effect of cytotoxicity of the test formulation was tested by treating the cells with different concentrations of the test formulation in EMEM medium.
Cytotoxicity by MTT assay
HepG2 cells were seeded in 96-well plates (at 1 X 104 cells/ well/180µl) of growth medium. Further, the cells were then incubated overnight under standard growth conditions so as to allow the cell recovery and exponential growth. The cells were then treated with positive control and Biofield Energy Treated and untreated test formulation and incubated in a CO2 incubator at 37°C, 5% CO2 , and 95% humidity. After 24 hours of incubation, the plate was taken out and 20µL of 5mg/ml of MTT was added to all the wells. The absorbance of each well was read at 540nm using Synergy HT microplate reader (SIAFRT/Synergy HT multimode reader, US), which was presented as the percentage cell growth corresponding to each treatment group. The effect of the test formulation on cell viability was determined as:
% Cell viability=100-%cytotoxicity-------------(1)
Where % cytotoxicity = [(O.D. of control cells-O.D. of cells treated with the test formulation)/O.D. of control cells]* 100.
Assessment of cell viability against oxidative damage
HepG2 cells were plated in 96-well plates at the density corresponding to 1 X 104 cells/well followed by overnight incubation in CO2 incubator at 37 °C, 5% CO2 , and 95% humidity. After overnight incubation, the cells were treated with Biofield Energy Treated and untreated test formulation at non-cytotoxic concentrations. After 24 hours of pre-treatment, the cells were treated with hydrogen peroxide (H2 O2 , 20mM) for 2 hours to induce oxidative stress. The untreated cells served as a control, while cells treated with H2 O2 alone served as a negative control. MTT assay was performed for calculation of percentage cell viability using the following formula:
% Cell viability = 100 – [(1-X/R)*100] ---------- (2)
Where, X = OD of wells corresponding to treated cells
R = OD of untreated cells (Cells maintained in growth medium only)
Determination of Intracellular lipid peroxidation (LPO) by TBARS method
HepG2 cells at the concentration corresponding to 5 X 105 cells/ well were plated in 6-well plates followed by overnight incubation in CO2 incubator at 37°C, 5% CO2 , and 95% humidity. After 24 hours of pre-treatment with the test formulation, the above cells were treated with H2 O2 (20mm) for 2 hours to induce oxidative stress. The cells treated with H2 O2 alone served as negative control. After incubation, the cell lysates were prepared by freeze-thaw lysis. The extent of lipid peroxidation was estimated in above cell lysates by measuring malondialdehyde (MDA) using the thiobarbituric acid reactive substances (TBARS) assay kit as per the manufacturer’s protocol. 100µL of sample or standard was mixed with 100µL of TCA assay reagent and 800µL of color reagent (thiobarbituric acid assay reagent) was added to the mix following by heating and cooling on ice bath for ten minutes. Absorbance was read at 540nm using Synergy HT microplate reader. The average of absorbance values corresponding to each standard and sample were normalized with blank absorbance. MDA levels of each sample were calculated from the standard curve. The percentage decrease in MDA levels with respect to H2O2 treatment was calculated as-
% Decrease of MDA = [(MDAH2O2- MDAsample)/ MDAH2O2]* 100 ---------- - (3)
Determination of intracellular superoxide dismutase (SOD) enzyme activity
HepG2 cells were counted using hemocytometer and plated in 6-well plates at the density corresponding to 5 X 105 cells/well followed by overnight incubation in CO2 incubator at 37 °C, 5% CO2, and 95% humidity. Further, the cells were treated with Biofield Energy Treated and untreated test formulation at the non-cytotoxic concentrations. Absorbance was read at 450nm using Synergy HT microplate reader. SOD activity of the samples was calculated using linear regression equation of the standard curve using equation 4.
% Increase of SOD = [(SODsample- SODH2O2)/ (SODuntreated-SODH2O2)]*100 ---------- (4)
Determination of catalase enzyme activity
HepG2 cells (5 X 105 cells/well) were plated in 6-well plates followed by overnight incubation. Further, cells were treated in different groups by test formulation at non-cytotoxic concentrations. After 24 hours of pre-treatment, cells were treated with hydrogen peroxide (H2O2 , 20mm) for 2 hours to induce oxidative stress. After incubation, cell lysates were prepared by freeze-thaw lysis. Catalase activity of the cells was assessed using Cayman catalase assay kit as per the manufacturer’s protocol. 20µL of standard or test formulation was added to the mixture of 100µL assay buffer and 30µL methanol. The reaction was initiated by adding 20µL H2 O2 and after 20 minutes of incubation, 30µL potassium hydroxide was added to terminate the reaction followed by the addition of 30µL catalase purpald. After an additional 10 minutes of incubation, 10µL potassium periodate was added and the absorbance was read after 5 minutes at 540nm using Synergy HT microplate reader. CAT activity was calculated from the formaldehyde concentration of the samples and percentage increase in the CAT activity with respect to H2O2 was calculated as-
% Increase of CAT = [(CATsample- CATH2O2)/CATH2O2]*100 ---------- (5)
Free radical scavenging activity-ABTS assay (Cell-free assay)
The ABTS radical scavenging activity of Biofield Energy Treated and untreated test formulation was determined by the method of Auddy et al. [31]. The ABTS reagent was kept in dark at room temperature for 12-16 hours before use to generate ABTS radical (ABTS•+). In brief, the total reaction mixture containing, the non-cytotoxic concentration of test formulation, and ABTS radical solution were mixed and immediately read at 734nm using a Synergy HT microplate reader. L-ascorbic acid was used as a reference standard. The plates were kept for 15 minutes at 37 °C. The experiment of free radical scavenging activity was carried out in triplicates by calculating % inhibition in absorbance as
ABTS radical scavenging activity (% Inhibition) = [(Ac-As)/ Ac]*100 ---------- (6)
Where, Ac stands for the absorbance of blank and as denote for the absorbance of sample.
Statistical analysis
All the data were expressed as percentage and performed oneway analysis of variance (ANOVA) followed by Dunnett’s test for multiple group comparison and Student’s t-test for two groups comparison. Statistical significance was considered at p≤0.05.
Results and Discussion
Cell viability using MTT assay
Figure 1: MTT assay in HepG2 cells after 24-hours of treatment with different test formulation concentrations and positive control. The absorbance was taken at 540 nm using Synergy HT microplate reader.

The cell viability results are summarized in (Figure 1). The results showed that all the tested concentrations have shown significant cell viability with more than 70% results. However, Biofield Energy Healing based Test Formulation significantly improved the cell viability at 10.41 and 1.041µg/ml concentrations as compared with the untreated test formulation. The cell viability in groups such as untreated cells was 100% while quercetin group at 100, 10, 1, 0.1, 0.01mM showed 146.1%, 70.24%, 108.88%, 81.14%, and 74.39% cell viability, respectively. The novel test formulation was tested at various concentrations ranging from 0.05 to 10.41µg/ ml, which were found safe and nontoxic. These concentration ranges were selected for the estimation of anti-oxidation activity in HepG2 cells. Out of the tested concentrations, the cell viability was increased by 42.39% and 11.93% in the Biofield Energy Treated test formulation i.e. at 1.04 and 10.4µg/ml, respectively. However, all the tested concentrations of test formulation have shown more than 70% cell viability. Cell viability can be effectively measured using MTT assay. The principle of MTT assay is based on cell growth and metabolic activity [32]. Thus, Biofield Energy Treated Test formulation is defined to have more metabolic activity as compared with the untreated test formulation
Effect of the test formulation for protection of cell viability against oxidative damage
Figure 2:The effect of the test formulation on HepG2 cells for cell viability after induction with H2O2. All the groups were challenged with H2O2 except baseline control (untreated cells group).
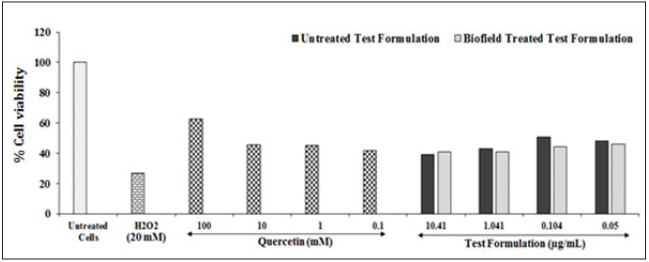
Effect of the Test Formulation for protection of cell viability against oxidative damage is shown in (Figure 2). The cells were challenged with H2O2 the percent cell viability was significantly decreased and reached to level 26.69% in the negative control group due to the generation of oxidative stress compared to the baseline control group. The reference item, quercetin showed significantly increased cell viability and showed percentage as 62.80%, 45.45%, 45.06%, and 41.87% at the concentration of 101, 10, 1, and 0.1 mm, respectively compared to the negative control group. [33], proved quercetin, a plant-derived aglycone a robust antioxidant activity as a nutritional supplement, which has various beneficial effects against a variety of diseases, including cancer [33]. Besides, the percent cell viability in HepG2 cells was significantly altered in the test formulation, while it was increased by 1.63% in the Biofield Energy Treated test formulation at 10.41µg/ml as compared with the untreated test formulation group.
Assessment of intracellular lipid peroxidation
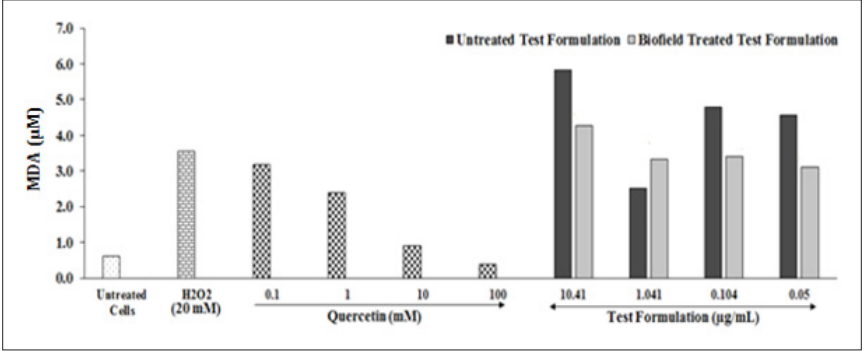
The level of LPO was measured among various Biofield Energy Treated test formulation at different concentrations under the stimulation H2O2. The results of the LPO activity was presented as malondialdehyde (MDA, µM) in (Figure 3). H2O2group showed a significantly increased level of LPO by 490%, while positive control quercetin at 100, 10, 1, and 0.1µM showed a significant reduced level of LPO activity by 89.21%, 74.69%, 31.12%, and 10.37%, respectively as compared with negative control (H2O2) group. Further, the test formulation at all the tested concentrations showed significantly reduced level of LPO as compared with the untreated test formulation. However, Biofield Energy Treated Test formulation showed 26.46%, 29.02%, and 32.16% reduced level of LPO at 10.41, 0.104, and 0.05µg/ml, respectively with respect to the untreated test formulation. Lipid peroxidation is defined as the process of oxidative damage, which affects cellular membranes, lipoproteins, and other related molecules containing the lipids in accordance with the oxidative stress. Cellular membrane lipids represent most often substrates of oxidative attack and is regarded as one the vital step in the pathogenesis of various disease states in adult and infant patients. This process would lead to various diseases such as atherosclerosis, inflammatory bowel disease, retinopathy of prematurity (ROP), borderline personality disorder (BPD), asthma, Parkinson’s disease, kidney damage, preeclampsia and many more [34-36]. Therefore, it is assumed that The Trivedi Effect®-Biofield Energy Treated novel test formulation is a powerful antioxidant agent and prevent the oxidation that leads to free radicals and various related diseases.
Figure 3: The effect of the biofield energy treated novel test formulation on HepG2 cells for the assessment of LPO activity in terms of lipid peroxide end product, malondialdehyde (MDA) under the induction of H2O2. All the groups were challenged with H2O2 except baseline control (untreated cells group).
Effect of the biofield energy treated test formulation on SOD activity
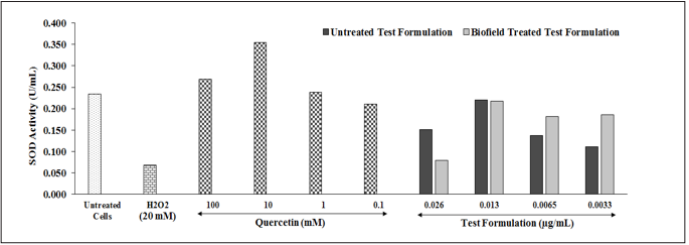
Intracellular SOD enzyme activity was measured among various Biofield Energy Treated test formulation concentrations under the influenced of hydrogen peroxide in HepG2 cells is shown in (Figure 4). The SOD activity in terms of U/ml was calculated and presented in different groups, which was compared with respect to untreated test formulation. H2O2 group significantly reduced the SOD activity, while positive control quercetin at 100, 10, 1, and 0.1mm showed significant improved SOD activity by 120.78%, 172.54%, 102.49%, and 85.66%, respectively as compared with negative control (H2O2) group. Further, the test formulation at all the tested concentrations showed a significant improved level of SOD. However, Biofield Energy Treated Test formulation showed 26.57% and 45.55% increased SOD activity at 0.0065 and 0.0033 µg/mL, respectively. SOD enzyme is considered as an important antioxidant defense mechanism in all living cells which are exposed to oxygen. It possess a powerful anti-inflammatory activity against chronic inflammation such as colitis. SOD enzyme supplement treatment reduced the reactive oxygen species generation, oxidative stress and inhibits the endothelial activation. Therefore, it is assumed that The Trivedi Effect®-Biofield Energy Treated novel test formulation must be a powerful antioxidant, which may be highly significant in new therapies for the treatment of inflammatory bowel disease and other inflammatory diseases.
Figure 4: The effects of the biofield energy treated novel test formulation on HepG2 cells for the assessment of SOD enzyme activity under the influence of hydrogen peroxide (H2O2). All the groups were challenged with H2O2 except baseline control.
Assessment of biofield energy treated test formulation on catalase enzyme activity
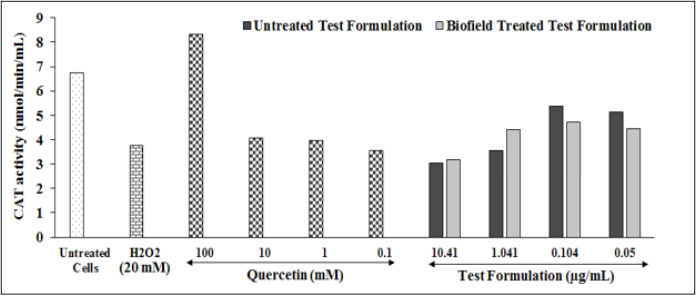
Catalase enzyme activity was measured among various Biofield Energy Treated test formulation at different concentrations after challenged with hydrogen peroxide in HepG2 cells. The results of CAT activity in terms of nmol/min/mL are represented in (Figure 5). H2O2 group showed a significantly reduced level of CAT activity by 44.28%, while positive control quercetin at 100 and 10mm showed significant improved CAT activity by 121.6% and 8.26%, respectively as compared with negative control (H2O2) group. Further, the test formulation at all the tested concentrations showed a significant improved level of CAT enzyme. However, Biofield Energy Treated Test formulation showed 4.77% and 28.57% increased CAT activity at 10.41 and 1.04µg/ml, respectively. CAT is defined as the longevity enzyme, scientifically-proven powerful antioxidant activity that prevent free radical damage to the body. CAT has many health benefits such as powerful antioxidant support, anti-aging and antidegenerative effects, it could increase the lifespan, fat reduction, and also helps in prevention of DNA damage [37]. Therefore, it is assumed that The Trivedi Effect®-Biofield Energy Treated novel test formulation is a powerful antioxidant agent and worked as free radical scavenging agent against many diseases.
Figure 5: The effects of the biofield energy treated novel formulation on HepG2 cells for the assessment of CAT enzyme activity under the influence of H2O2. All the groups were challenged with H2O2 except baseline control.
Assessment of free-radical scavenging activity by ABTS assay (Cell free assay)
The relative antioxidant ability of the test formulation to scavenge the radical ABTS+ has been compared with the standard ascorbic acid along with untreated test formulation. The free radical scavenging activity was tested using ABTS assay in various Biofield Energy Treated test formulation group at different concentrations after challenged with hydrogen peroxide in HepG2 cells. The results of ABTS assay was presented as percentage inhibition in (Figure 6). The positive control group, ascorbic acid at 200, 400, 600, and 800µg/ml showed significant increased inhibition of ABTS radical by 13.91%, 27.83%, 60.55%, and 77.52%, respectively as compared with the baseline group. In addition, the test formulation at all the tested concentrations showed a significant reduction in the level of ABTS free radical as compared with the untreated test formulation. However, Biofield Energy Treated Test formulation showed further 5.8% and 9.8% inhibition at 0.05% and 1% test formulation concentration, respectively with respect to the untreated test formulation. The ABTS radical cation scavenging activity also reflects the hydrogen-donating ability [38,39]. This suggest that Biofield Energy Treated Test formulation have significant ability to quench the free radicals (ABTS•+). Since, the Biofield Energy Treated Test formulation have the ability to scavenge free radicals, thereby preventing the lipid oxidation via a chain breaking reaction, which could serve as potential nutraceuticals against various inflammatory disorders.
Figure 6: Free-radical scavenging activity using ABTS assay of the biofield energy treated novel test formulation on HepG2 cells
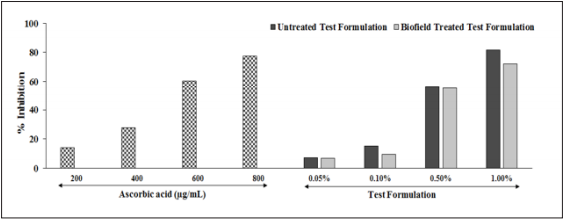
Thus, it can be suggested that the novel proprietary Biofield Energy Treated test formulation significantly improved antioxidant profile along with total antioxidant property using ABTS assay. Thus, overall data suggested the use of test formulation against various autoimmune disorders, anti-inflammatory diseases, antiaging, and many more.
Conclusion
On the basis of study findings, the MTT assay data suggested that the Biofield Energy Treated test formulation was found safe and non-toxic at the tested concentration upto 10.41µg/ml. Additionally, the Biofield Energy Treated test formulation was significantly protected the HepG2 cells under hydrogen peroxide (H2O2) induced oxidative cell damage. The level of SOD was significantly increased by 26.57% and 45.55% in the Biofield Energy Treated Test formulation at 0.0065 and 0.0033µg/ml, respectively as compared with the untreated test formulation. CAT enzyme activity was also significantly increased by 28.57% at 1.04µg/ml as compared with the untreated test formulation. However, LPO was significantly decreased by 26.46%, 29.02%, and 32.16% at 10.41, 0.104, and 0.05µg/ml, respectively with respect to the untreated test formulation. The total antioxidant capacity using ABTS assay showed that the Biofield Energy Treated Test formulation showed 9.8% inhibition of ABTS radicle (radical scavenging activity) at 1% with respect to the untreated test formulation. On the basis of experimental results, the Biofield Energy Treated novel proprietary test formulation has significantly protected the HepG2 cells from oxidative stress induced by H2O2. Therefore, The Trivedi Effect®- Biofield Energy Healing Treated test formulation would be used against various autoimmune disorders (rheumatoid arthritis), anti-inflammatory diseases (Irritable Bowel Syndrome, Ulcerative colitis and Crohn’s disease), antiaging, and oxidative and freeradicals related diseases such as atherosclerosis, Alzheimer’s disease, cancer, ocular disease, diabetes, and motor neuron disease etc. Moreover, it can also be used for improving the antioxidant capability of different organs/tissues that might be useful against to manage different type of stress conditions and improve body’s detoxification process
Acknowledgement
The authors are grateful to Dabur Research Foundation, Trivedi Science, Trivedi Global, Inc., and Trivedi Master Wellness for their support throughout the work.
Conflict of Interest
Authors declare no potential conflict of interest
References
- Nita M, Grzybowski A (2016) The role of the reactive oxygen species and oxidative stress in the pathomechanism of the age-related ocular diseases and other pathologies of the anterior and posterior eye segments in adults. Oxid Med Cell Longev.
- Schieber M, Chandel NS (2014) ROS function in redox signaling and oxidative stress. Curr Biol 24(10): R453-R462.
- Uttara B, Singh AV, Zamboni P, Mahajan RT (2009) oxidative stress and neurodegenerative diseases: A review of upstream and downstream antioxidant therapeutic options. Curr Neuropharmacol 7(1): 65-74.
- Pham-Huy LA, Hua He, Pham-Huy C (2008) Free radicals, antioxidants in disease and health. Int J Biomed Sci 4(2): 89-96.
- cdc.gov/nchs/data/nhsr/nhsr012.pdf
- Astin JA, Pelletier KR, Marie A, Haskell WL (2000) Complementary and alternative medicine use among elderly persons: One-year analysis of a blue shield medicare supplement. J Gerontol A Biol Sci Med Sci 55(1): M4-M9.
- Müller O, Krawinkel M (2005) Malnutrition and health in developing countries. CMAJ 173(3): 279-286.
- Li P, Zheng Y, Chen X (2017) Drugs for autoimmune inflammatory diseases: From small molecule compounds to anti-TNF biologics. Front Pharmacol 8: 460.
- Müller O, Krawinkel M (2005) Malnutrition and health in developing countries. CMAJ 173(3): 279-286.
- Gautam SC, Gao X, Dulchavsky S (2007) Immunomodulation by curcumin. Adv Exp Med Biol 595: 321-341.
- Lukac N, Massanyi P (2007) Effects of trace elements on the immune system. Epidemiol Microbial Immunol 56(1): 3-9.
- Galland L (1988) Magnesium and immune function: an overview. Magnesium 7(5-6): 290‐
- Frass M, Strassl RP, Friehs H, Mullner M, Kundi M, et al. (2012) Use and acceptance of complementary and alternative medicine among the general population and medical personnel: A systematic review. Ochsner J 12(1): 45-56.
- Trivedi MK, Mohan TR (2016) Biofield energy signals, energy transmission and neutrinos. American Journal of Modern Physics 5(6): 172-176.
- Trivedi MK, Branton A, Trivedi D, Nayak G, Mondal SC, et al. (2015) Antimicrobial sensitivity, biochemical characteristics and biotyping of Staphylococcus saprophyticus: An impact of biofield energy treatment. J Women’s Health Care 4: 271.
- Trivedi MK, Patil S, Shettigar H, Mondal SC, Jana S (2015) In vitro evaluation of biofield treatment on Enterobacter cloacae: Impact on antimicrobial susceptibility and biotype. J Bacteriol Parasitol 6: 241.
- Trivedi MK, Patil S, Shettigar H, Mondal SC, Jana S (2015) Evaluation of biofield modality on viral load of Hepatitis B and C Viruses. J Antivir Antiretrovir 7: 83-88.
- Trivedi MK, Branton A, Trivedi D, Nayak G, Mondal SC, et al. (2015) Antibiogram of biofield-treated Shigella boydii: Global burden of infections. Science Journal of Clinical Medicine 4: 121-126.
- Trivedi MK, Branton A, Trivedi D, Nayak G, Mondal SC, et al. (2015) Evaluation of antibiogram, genotype and phylogenetic analysis of biofield treated Nocardia otitidis. Biol Syst Open Access 4: 143.
- Trivedi MK, Branton A, Trivedi D, Nayak G, Charan S, et al. (2015) Phenotyping and 16S r DNA analysis after biofield treatment on Citrobacter braakii: A urinary pathogen. J Clin Med Genom 3: 129.
- Peoples JJ, Trivedi MK, Branton A, Trivedi D, Nayak G, et al. (2017) Skin rejuvenating effect of consciousness energy healing treatment based herbomineral formulation. American Journal of Plant Biology 2: 77-87.
- Smith DM, Trivedi MK, Branton A, Trivedi D, Nayak G, et al. (2017) Skin protective activity of consciousness energy healing treatment based herbomineral formulation. Journal of Food and Nutrition Sciences 5: 86-95.
- Trivedi MK, Branton A, Trivedi D, Nayak G, Gangwar M, et al. (2015) Analysis of genetic diversity using simple sequence repeat (SSR) markers and growth regulator response in biofield treated cotton (Gossypium hirsutum L.). American Journal of Agriculture and Forestry 3(5): 216-221.
- Trivedi MK, Branton A, Trivedi D, Nayak G, Gangwar M, et al. (2015) Evaluation of vegetative growth parameters in biofield treated bottle gourd (Lagenaria siceraria) and okra (Abelmoschus esculentus). International Journal of Nutrition and Food Sciences 4: 688-694.
- Trivedi MK, Branton A, Trivedi D, Nayak G, Balmer AJ, et al. (2016) Evaluation of pro-inflammatory cytokines expression in mouse splenocytes after incubation with the biofield energy healing based herbomineral formulation: Influence of the Trivedi Effect®. American Journal of Bioscience and Bioengineering 4(5): 87-97.
- Trivedi MK, Branton A, Trivedi D, Nayak G, Ellis MP, et al. (2016) Evaluation of pro-inflammatory cytokines expression in mouse splenocytes after co-incubation with the biofield energy treated formulation: Impact of the Trivedi Effect®. International Journal of Biomedical Science and Engineering 4(5): 40-49.
- Trivedi MK, Patil S, Shettigar H, Bairwa K, Jana S (2015) Spectroscopic characterization of biofield treated metronidazole and tinidazole. Med Chem 5: 340-344.
- Trivedi MK, Patil S, Shettigar H, Bairwa K, Jana S (2015) Effect of biofield treatment on spectral properties of paracetamol and piroxicam. Chem Sci J 6: 98.
- Trivedi MK, Tallapragada RM, Branton A, Trivedi D, Nayak G, et al. (2015) Evaluation of atomic, physical, and thermal properties of bismuth oxide powder: An impact of biofield energy treatment. American Journal of Nano Research and Applications 3: 94-98.
- Trivedi MK, Patil S, Nayak G, Jana S, Latiyal O (2015) Influence of biofield treatment on physical, structural and spectral properties of boron nitride. J Material Sci Eng 4: 181.
- Gangwar M, Gautam MK, Sharma AK, Tripathi YB, Goel RK, et al. (2014) Antioxidant capacity and radical scavenging effect of polyphenol rich mallotus philippenensis fruit extract on human erythrocytes: An in vitro Scientific World Journal.
- Rai Y, Pathak R, Kumari N, Sah DK, Pandey S, et al. (2018) Mitochondrial biogenesis and metabolic hyperactivation limits the application of MTT assay in the estimation of radiation induced growth inhibition. Sci Rep 8(1):1531.
- Zhang M, Swarts SG, Yin L, Liu C, Tian Y, et al. (2011) Antioxidant properties of quercetin. Adv Exp Med Biol 701: 283-289.
- Vasilaki AT, McMillan DC (2011) Lipid Peroxidation. In: Schwab M (eds), Encyclopedia of Cancer. Springer, Berlin, Heidelberg, Germany.
- Moller P, Loft S (2010) Oxidative damage to DNA and lipids as biomarkers of exposure to air pollution. Environ Health Perspect 118 (8): 1126-1136.
- Negre-SA, Coatrieux C, Ingueneau C, Salvayre R (2008) Advanced lipid peroxidation end products in oxidative damage to proteins. Potential role in diseases and therapeutic prospects for the inhibitors. Br J Pharmacol 153(1): 6-20.
- Djordjevic VB (2004) Free radicals in cell biology. Int Rev Cytol 237: 57-89.
- Re R, Pellegrini N, Proteggente A, Pannala A, Yang M, et al. (1999) Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic Biol Med 26(9-10): 1231-1237.
- Dong JW, Cai L, Xing Y, Yu J, Ding ZT (2015) Re-evaluation of ABTS*+ assay for total antioxidant capacity of natural products. Nat Prod Commun 10 (12): 2169-2172.
© 2019 Mahendra Kumar Trivedi. This is an open access article distributed under the terms of the Creative Commons Attribution License , which permits unrestricted use, distribution, and build upon your work non-commercially.
 a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in
a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in 







.jpg)






























 Editorial Board Registrations
Editorial Board Registrations Submit your Article
Submit your Article Refer a Friend
Refer a Friend Advertise With Us
Advertise With Us
.jpg)






.jpg)














.bmp)
.jpg)
.png)
.jpg)










.jpg)






.png)

.png)



.png)






