- Submissions

Full Text
Modern Applications in Pharmacy & Pharmacology
Synergistic Bioactivity of Activated Carbon (Charcoal), Anogeissus Leiocarpus and Nauclea Latifolia on Selected Clinical Isolates
Oludare Temitope Osuntokun1*, Taiye A Jemilaiye1 and AM Yusuf-Babatunde2
1 Department of Microbiology, Nigeria
2 Department of Pharmaceutical Technology, Nigeria
*Corresponding author: Oludare Temitope Osuntokun, Department of Microbiology, Nigeria
Submission: May 14, 2018;Published: December 03, 2018

ISSN 2637-7756Volume2 Issue3
Abstract
This study evaluated the Synergistic bioactivity of activated carbon (charcoal), Angliss’s lei carpus, Naugle latifoliate against five clinical bacteria and fungi isolates (Gram positive and Gram-negative bacteria). Angliss’s eriocarpin (African Birch; Bambara: Naglaa) is a tall deciduous tree native to savannah of tropical Africa. One of the most widely used substance for water purification is activated carbon due to its large surface area and high adsorption capacity. The bacteria are Escherichia coli, Staphylococcus aureus, Bacillus subtilis, Proteus mirabilis and Klebsiella pneumoniae. Selected fungal isolates which include; Aspergillus flavus, Aspergillus Niger, Fusarium solani, Candida albican and S. cerevisae. Antimicrobial screening of crude ethanolic leaf extract of Anogeisssus leiocarpus and ethanolic stem bark extract of Naucle latifolia was carried out using agar well diffusion. The crude extract from both Anogeisssus leiocarpus leaf shows high antimicrobial activities while the Naucle latifolia stem bark extract showed moderate antibacterial and antifungal activity. The synergistic effect of the Anogeisssus leiocarpus leaf and activated carbon shows comparatively higher antimicrobial activities than individual plant and carbon. Highest diameter zones of inhibition were observed from the synergy of A.
Anogeisssus leiocarpus leaf and activated carbon against E-coli with 24mm at 100mg/ml, while the least zone of inhibition is observed from the Anogeisssus leiocarpus extract against K. pneumoniae with 18mm at 100mg/ml and 11mm at 12.5mg/ml. The Minimum inhibitory concentration value of Anogeisssus leiocarpus leaf against the bacterial isolates is 12.5mg/ml, while the Minimum bactericidal concentration of activated carbon against the isolates is 25mg/ml concentration. Phytochemical analysis reveals the presence of Alkaloid, Cardiac glycoside,Steroids, Tannin, Saponin, Flavonoid, Anthraquinone, Phenol and reducing sugar. Due to the data recorded from this research work, it can be concluded that, activated carbon can be used in synergy with he mentions medicinal plant (Anogeissus leiocarpus & Nauclea latifolia) for the effective treatment of public water source and recalcitrant clinical isolates.
Keywords: Anogeissus leiocarpus; Nauclea latifolia; Activated carbon; Synergistic bioactivity
Introduction
Activated carbon is widely used for water purification due to its large surface area and high adsorption capacity. Activated carbon has proven to remove bacteria like Pseudomonas aeruginosa and Escherichia coli from fresh and potable water systems. Despite electrostatic repulsion between negatively charged microorganisms and carbon surfaces, microorganisms attach to activated carbon particles through strong Lifshitz vander Waals forces. Potable water systems are considered low in ionic strength so electrostatic interactions can offer the possibility of enhancing the efficacy of activated carbon to remove microorganisms from water by positive charge modification of the carbon surfaces. Once there is a charge reversal, the electrostatic attraction between negatively charged microbial cell surfaces and positively modified carbon particles will be strong [1].
Moreover, modification in the activated carbon particles by coating with a quaternary ammonium compound gives the activated carbon particles bactericidal properties and decreases the possibility of biofilm growth. In addition to the microorganisms charge, the hydrophobicity of the surfaces that come in contact with microbes is important in adhesion Activated carbon have attracted great interest in their development as potential antibacterial drugs [2], which has also been reported that many biophysical interactions occur between Activated carbon and bacteria including biosorption, decomposition and cellular uptake, with effects bacterial cell membrane damage and toxicity [3]. Anogeissus leiocarpa (African Birch; Bambara: ngálǎma) is a tall deciduous tree native to savannas of tropical Africa. It is the sole West African species of the genus Anogeissus, a genus otherwise distributed from tropical central and east Africa through tropical Southeast Asia. Callicarpa germinates in the new soils produced by seasonal wetlands and grows at the edges of the rainforest, although not in the rainforest, in the savanna, and along riverbanks forming gallery forests. The tree flowers in the rainy season, from June to October. The seeds, winged samaras, are dispersed by ants [4].
Anogeissus leiocarpus is a deciduous tree species that can grow up to 15-18m of height and measure up to 1m diameter. Bark greyish, scaly. Branches often drooping and slender, leaves alternate, ovate-lanceolate in shape, 2-8cm long and 1.3-5cm across. The leaves are acute at the apex and attenuate at the base, pubescent beneath. Inflorescence globose heads, 2cm across, yellow; the flowers are bisexual, petals absent. Fruits are globose cone like heads; each fruit is broadly winged, dark grey, 3cm across. It can reproduce by seeds as well as vegetative propagation [5]. The leaves of the plant are used externally as a decoction in the eastern part of Nigeria for the treatment of skin diseases and the itch of psoriasis. The powdered bark is applied to wounds, sores, boils, cysts and diabetic ulcers with good results. The powdered leaf has also been mixed with ‘green clay’ and applied as an unusual face mask for serious blackheads. The infusion and decoctions are used as cough medicine, the pulped roots are applied to wounds and ulcers, the powdered bark is also rubbed to reduced tooth ache on gums, it is also used as vermifuges and the leaves decoction is used for washing and fumigation [6].
Nauclea is a genus of flowering plants in the Rubiaceae family. The species are evergreen trees or shrubs that are native to the pale tropics. The terminal vegetative buds are usually strongly flattened. The generic name is derived from the Ancient Greek words naus, meaning “ship” and kleio, meaning “to close”. It refers to the resemblance of the cells of the capsule to a ship’s hull. Nauclea latifolia commonly known as a pin-cushion tree is a straggling shrub native to the tropical Africa and Asia, commonly found in the tropical forests. Nauclea latifolia commonly known as a pin-cushion tree is a straggling shrub native to the tropical Africa and Asia, commonly found in the tropical forests. In Nigeria, it is commonly and abundantly found in Akwa Ibom and Cross River States where it is called “Mbom-ibong”, in most parts of the Northern Nigeria, it is called “Tabasiya”. In Easten Nigeria, the Igbo’s called it “Ubulinu” [7]. Nearly all the plant’s parts, but scarcely the roots; have been widely exploited and reported to be useful in the treatment and remedy of diverse infections and ailments such as stomachpains, constipation, tuberculosis, malaria, gonorrhea, sores, piles, hypoglycemia, sleeping sickness, gastrointestinal tract disorders, prolonged menstrual flow, hypertension, fever tonic, chewing-stock for tooth aches, dental-caries, septic-mouth, jaundice, infantilegastroenteritis, including dysentery and diarrhea [8].
Materials and Methods
Collection of Nauclea latifolia and Anogeissus leiocarpus
The stem bark and leaves of Nauclea latifolia and Anogeissus leiocarpus used in this study were collected from fresh water swamp forest in Akungba-Akoko, Ondo State, Nigeria and were authenticated by taxonomist in the Department of Plant Science and Biotechnology, Adekunle Ajasin University, Akungba-Akoko, Ondo State, Nigeria.
Preparation of Nauclea latifolis and Anogeissus leiocarpus
The collected stem bark and leaves samples of Nauclea latifolia and Anogeissus leiocarpus were washed thoroughly with sterile distilled water and air dried at room temperature for two weeks. The stem bark and leaves of Nauclea latifolia and Anogeissus leiocarpus were crushed and blended into fine particles.
Extraction of Nauclea latifolia and Anogeissus leiocarpus
500g of each ground plant parts were soaked in 1500ml of dichloromethane and intermittently shaken for 7 days. The resulting mixtures were filtered using sterile Whatman filter papers. The filtrates collected were concentrated using rotary evaporator and the crude extracts thus obtained were kept in the refrigerator [9].
Standardization of Nauclea latifolia and Anogeissus leiocarpus
At aseptic condition, 1g of each extract was measured and dissolved in 2.5ml of Dimethyl soapboxed (DMSO) and 7.5ml of sterile distilled water to obtain a stock concentration of 100mg/ ml. This stock was further reconstituted to 50mg/ml, 25mg/ml, 12.5mg/ml and 6.25mg/ml concentrations using the formula C1V1=C2V2 [10].
Collection of clinical isolates
The clinical isolates used were strains of pathogenic bacteria obtained from the stock culture of organisms at the Microbiology laboratory, Adekunle Ajasin University, Akungba-Akoko, Ondo State. The bacteria used were Salmonella typhi, Escherichia coli, Klebsiella pneumoniae, Bacillus subtilis, Proteus mirabilis and Staphylococcus aureus. The fungal isolates are Fusarium solani, Aspergillus flavus, Candida albican, Saccharomyces cerevisiae and Aspergillus Niger. The microorganisms were subculture into nutrient broth 24 hours prior to bioassay of the extract Figure 1.
Figure 1:Anogeissus leiocarpus.

Preparation of inoculum
A 24 hours broth culture of the microorganism was diluted and standardized to 10-4 dilution. 0.1ml of the broth culture was pipetted into 9.9ml of sterile distilled water contained in a test tube and was mixed properly. This was repeated by pipetting from the first dilution and adding into another 9.9ml sterile distilled water [10].
Antimicrobial assay of the extracts (Nauclea latifolia and Anogeissus leiocarpus)
Figure 2:Nauclea latifolia.

Standard agar well diffusion method was employed for the antimicrobial testing. About 50μl each of the constituted extracts previously diluted to 50mg/ml, 25mg/ml, 12.5mg/ml and 6.25mg/ ml concentration was introduced into four different wells bore on the agar (Mueller-hinton agar) plate inoculated with the test isolates. The plates were incubated at 37 ℃ for 24 hours for bacteria and 25 ℃ for 72 hours for fungi Levofloxacin 50mg/ml was used as the control. After the period of incubation, the antimicrobial activity was expressed in terms of diameter zones of inhibition Figure 2.
Tube dilution method for assaying activated carbon
Concentrations of 100mg/ml, 50mg/ml, 25mg/ml and 12.5mg/ ml of the activated carbon were constituted using Sterile Mueller- Hinton broth. Standardized inoculum was introduced into each test tube and incubated at 37 °C for 24 hours for bacteria and 25 °C for 72 hours for fungi. The tubes were then examined for turbidity indicating microbial growth.
Synergistic in-vitro (antibacterial and antifungal) activity of activated carbon, Nauclea latifolia and Anogeissus leiocarpus)
The plant extract and the activated carbon were mixed in ratio 1:1, 200mg/ml of the activated carbon and 200mg/ml of the plant extract (Anogeissus leiocarpus or Nauclea latifolia) were mixed together and homogenized. 50μl of each concentration of the constituted mixture was introduced into wells on Mueller Hinton agar plate and incubated as described above. Levofloxacin 50mg/ ml was used as the control. The zones of inhibition were measured to the nearest millimeter (mm) using a standard transparent meter rule. All experiments were performed in duplicates [10].
Determination of minimum inhibitory concentration
Minimum inhibitory concentration (MIC) was also measured. Dilution law was used to determine the concentration per plate. The working concentration was 0.25mg/ml, 0.5mg/ml, 1mg/ml, 2mg/ ml, 4mg/ml, 8mg/ml, and 16mg/ml. The dilution law; C1V1=C2V2 was employed to determine the quantity of the extracts to be added to the agar. The mixture of the agar and extract could solidify, and the standardized organisms were inoculated on the plates each. For this procedure, extracts were tested on the test fungal isolates to determine the minimum concentration of inhibition. The Petri dish containing the agar and extract mix strip with the organism was incubated at 37 °C for fungi and 24 °C for fungi and examined after 24 and 48 hours respectively. The lowest concentration of the extract at which there is inhibition of the organism growth is taken as the minimum inhibitory concentration (MIC) [10].
Determination of minimum fungicidal concentration
Minimum fungicidal concentration (MFC) was also measured. Dilution law was used to determine the concentration per plate. The working concentration was 0.25mg/ml, 0.5mg/ml, 1mg/ml, 2mg/ ml, 4mg/ml, 8mg/ml, and 16mg/ml. The dilution law; C1*V1 = C2*V2. While C1 is 100mg/ml and V2 is 20mls was employed to determine the quantity of the extracts to be added to the agar. The mixture of the agar and extract could solidify, and the standardized organisms were inoculated on the plates each. For this procedure, extracts were tested on the test fungal and fungal isolates to determine the minimum concentration of inhibition. The Petri dish containing the agar and extract mix strip with the organism was incubated at 37 °C for fungi and 24 °C for fungi and examined after 24 and 72 hours respectively. The lowest concentration of the extract at which there is inhibition of the organism growth is taken as the minimum inhibitory concentration (MIC) [10].
Qualitative phytochemical analysis of Nauclea latifolis and Anogeissus leiocarpus
Test for reducing sugars: One milliliter of the plant filtrate was mixed with Fehling A and Fehling B separately; a brown color with Fehling B and a green colour with Fehling A indicate the presence of reducing sugars.
Test for alkanol (TLC method): Wet the powdered test samples with a half diluted NH4OH and lixiviated with EtOAc for 24hr at room temperature. Separate the organic phase from the acidified filtrate and basify with NH4OH (pH 11-12). Then extract it with chloroform (3X), condense by evaporation and use for chromatography. Separate the Alkaloid spots using the solvent mixture chloroform and methanol (15:1). Spray the spots with Drageoir’s reagent. Orange spot shows a positive result [11].
Test for Anthraquinone: Borntrager’s test-Heat about 50mg of extract with 1ml 10% ferric chloride solution and 1ml of concentrated hydrochloric acid. Cool the extract and filter. Shake the filtrate with equal amount of diethyl ether. Further extract the ether extract with strong ammonia. Pink or deep red coloration of aqueous [12].
Test for cardiac glycosides: TLC method-Extract the powdered test samples with 70% EtOH on rotary shaker (180 thaws/min) for 10hr. Add 70% lead acetate to the filtrate and centrifuge at 5000rpm/10min. Further centrifuge the supernatant by adding 6.3% Na2CO3 at 10000rpm/10min. Dry the retained supernatant and re-dissolved in chloroform and use for chromatography. Separate the glycosides using EtOAc-MeOH-H2O (80:10:10) solvent mixture. The color and hRf values of these spots can be recorded under ultraviolet (UV254 nm) light [11].
Test for Flavonoid: TLC method - Extract 1g powdered test samples with 10ml methanol on water bath (60 °C/5min). Condense the filtrate by evaporation and add a mixture of water and EtOAc (10:1mL) and mix thoroughly. Retain the EtOAc phase and use for chromatography. Separate the Flavonoid spots using chloroform and methanol (19:1) solvent mixture. The color and hRf values of these spots can be recorded under ultraviolet (UV254nm) light [11].
Test for Phenol: Phenol test: Spot the extract on a filter paper. Add a drop of phosphomolybdic acid reagent and expose to ammonia vapors. Blue coloration of the spot, shows is a positive result [12].
Test for saponin: TLC method- Extract two grams of powdered test samples with 10ml 70% EtOH by refluxing for 10min. condense the filtrate, enrich with saturated n-BuOH, and mix thoroughly. Retain the butanol, condense and use for chromatography. Separate the Saponins using chloroform, glacial acetic acid, methanol and water (64:34:12:8) solvent mixture. Expose the chromatogram to the iodine vapors. The color (yellow) and hRf values of these spots were recorded by exposing chromatogram to the iodine vapour [11].
Test for steroid: TLC method: Extract two grams of powdered test samples with 10ml methanol in water bath (80 °C/15min). Use the condensed filtrate for chromatography. The sterols can be separated using chloroform, glacial acetic acid, methanol and water (64:34:12:8) solvent mixture. The color and hRf values of these spots can be recorded under visible light after spraying the plates with anisaldehyde-sulphuric acid reagent and heating (100 °C /6 min). The color (Greenish black to Pinkish black) and hRf values of these spots can be recorded under visible light [11].
Test for tannin: Braemer’s test 10% alcoholic ferric chloride will be added to 2-3ml of methanolic extract (1:1) Dark blue or greenish grey coloration of the solution [12,13].
Quantitative phytochemical analysis Nauclea latifolis and Anogeissus leiocarpus
Determination of saponins: About 20grams each of dried plant samples were ground and, put into a conical flask after which 100ml of 20% aqueous ethanol were added. The mixtures were heated using a hot water bath. At about 55 °C, for 4 hours with continuous stirring, after which the mixture was filtered, and the residue re-extracted with a further 200ml of 20% ethanol. The combined extracts were reduced to 40ml over a water bath at about 90 °C. The concentrate was transferred into a 250ml separatory funnel and 20rnl of diethyl ether were added and then shaken vigorously. The aqueous layer was recovered while the ether layer was discarded. The purification process was repeated three times. 60rnl of n-butanol were added. The combined n-butanol extracts were washed twice with 10ml of 5% aqueous sodium chloride. The remaining solution was heated in a water bath. After evaporation, the samples were dried in the oven to a constant weight; the saponin content was calculated as percentage of the starting material [14].
Determination of Flavonoids: About 10g of the plant sample were extracted repeatedly with 100ml of 80% aqueous methanol, at room temperature. The whole solution was filtered through Whatman filter paper No 42. The filtrate was later transferred into a crucible and evaporated into dryness over a water bath; the dry content was weighed to a constant weigh [14].
Estimation of Tannins: About 500 mg of the plant sample were weighed into a 50ml plastic bottle. 50ml of distilled water was added and shaken for 1 hour on a mechanical shaker. This was filtered into a 50ml volumetric flask and made up to the marked level. Then, 5ml of the filtrate was transferred into a test tube and mixed with 2ml of 0.1M FeCl in 0.1M Hcl and 0.008M potassium ferro cyanide. The absorbance was measured at 120nm within 10 minutes. The Tannins content was calculated using a standard curve of extract [14].
Estimation of Alkaloids: Five grams of the plant sample were weighed into a 250ml beaker and 200ml of 10% acetic acid in ethanol was then be added, the reaction mixture was covered and allowed to stand for 4 hours. This was filtered, and the extract will be concentrated on a water bath to one-quarter of the original volume. Concentrated ammonium hydroxide was added drop-wise to the extract until the precipitation is complete. The whole solution was allowed to settle, and the precipitate was collected, washed with dilute ammonium hydroxide and then filtered; the residue being the Alkaloid, which was dried and weighed to a constant mass [14].
Result
Table 1 Shows the antibacterial activities of activated carbon against all the selected clinical organisms using tube dilution method. B. subtilis and S. typhi were observed to have the highest susceptibility to the activated carbon with growth inhibition at 8mg/ ml 16mg/ml and 32mg/ml. S. aureus, P.mirabilis, K. pneumoniae showed relatively moderate susceptibility to this activated carbon with clear tube at 16mg/ml and 32mg/ml. From this study, S. aureus, P. mirabilis, K. pneumoniae were also observed to have the lowest susceptibility to the Activated carbon. Table 2 Shows the antifungal activities of activated carbon against all the selected fungal isolates using tube dilution method. Antifungal screening showed that Aspergillus Niger and Yeast were observed to have the highest susceptibility to the activated carbon with growth inhibition at 8mg/ml, 16mg/ml and 32mg/ml. F. solani, A. flavus, and C. albican showed the least susceptibility to this activated carbon with growth inhibition at 16mg/ml and 32mg/ml.
Table 1:Antibacterial activity of activated carbon using tube dilution method against selected clinical organism.
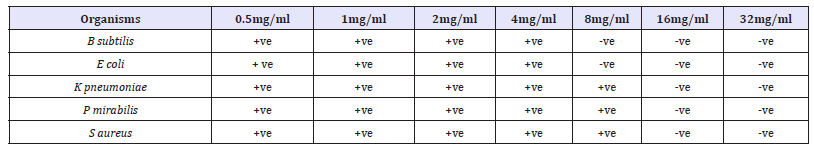
Key: -ve = Negative (No Growth), +ve = Positive (Growth)
Table 2:Antifungal activity of Activated carbon using tube dilution method against selected fungal isolates.

Key: -ve = Negative (No Growth), +ve = Positive (Growth)
Table 3:Zone of inhibition of leaf extract of Anogeissus leiocarpus on selected fungal isolates.
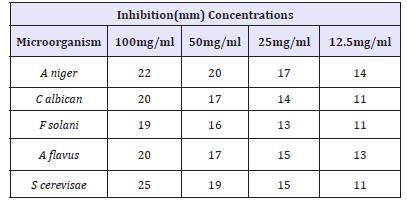
Table 3 shows the antifungal activity of the activity of Anogeissus leiocarpus leaf extract on selected clinical isolates. From this table, S. cerevisae. was shown to have the highest susceptibility with the 25mm zone of inhibition at 100mg/ml concentration, and 11mm zone of inhibition at a concentration of 12.5mg/ml. It was also observed from this table that Aspergillus Niger has a good susceptibility value of 22mm zone of inhibition at concentration of 100mg/ml. It was also observed, that C. albicans and A. flavus has 20mm and 19mm zones of inhibition respectively at concentrations of 100mg/ ml respectively, While F. solani as the lowest zone of inhibition with 19mm at a concentration of 100mg/ml.
Table 4 Shows the diameter (in mm) zones of inhibition of clinical isolates at different concentrations (100mg/ml, 50mg/ ml, 25mg/ml, 12.5mg/ml and 6.25mg/ml) of Nauclea latifolia leaf extract. The zones of inhibition were recorded as mean of two replicates. All the test organisms which include Bacillus subtilis, Salmonella typhi, Klebsiella pneumonia, and Proteus mirabilis were observed to be susceptible to the extract of Nauclea latifolia with 20.0mm zones of inhibition found in E-coli. Table 5 Shows the synergistic antifungal activity of Anogeissus leiocarpus leaf extract and activated carbon (ratio 1:1). From this table, A. flavus was shown to has the highest susceptibility value of 24mm zone of inhibition at 100mg/ml concentration, and 15mm zone of inhibition at a concentration of 12.5mg/ml. It was also observed from this table that. Aspergillus Niger and S. cerevisae has susceptibility value of 22mm zones of inhibition respectively 100mg/ml respectively, While C. albicans has the lowest zone of inhibition of 21mm at concentration of 100mg/ml.
Table 4:Antibacterial activity of Nauclea latifolia extract against clinical isolates.
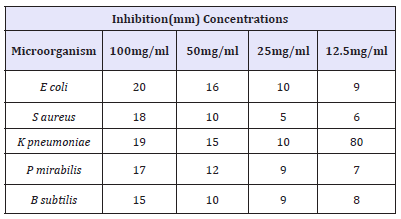
Table 5:Synergistic antifungal activity of Activated carbon and Anogeissus leiocarpus against selected fungal isolate.

Table 6:Synergistic Antibacterial activity of Nauclea latifolia Extract and activated carbon (Charcoal) against selected clinical isolates.

Table 6 Shows the diameter (in mm) of the zones of inhibition of clinical bacterial isolates at different concentrations (100mg/ ml, 50mg/ml, 25mg/ml, 12.5mg/ml and 6.25mg/ml) of activated carbon and Nauclea latifolia leaf extract. The zones of inhibition were recorded as mean of two replicates. All the test organism which include Bacillus subtilis, Salmonella typhi, Klebsiella pneumonia, Proteus mirabilis were discovered to be very susceptible to the extract of Nauclea latifolia, with very wide zone of inhibition. minimum inhibitory concentration (MIC) value of Anogeissus leiocarpus against the test bacteria used in the study. From this study, S. aureus, P. mirabilis and E-coli were the bacteria with the least MIC values, having their MIC values at 6.25mg/ml respectively, while B. subtilisi and K. pneumoniae were the test bacteria with the highest MIC value, having its MIC value at 12.5mg/ml. it was concluded that, the MIC values of 6.25mg/ml and 12.5mg/ml all the test organism will be inhibited.
Table 7:Minimum inhibitory concentration (MIC) of ethanol Anogeissus leiocarpus leaf Extract against selected clinical isolates.
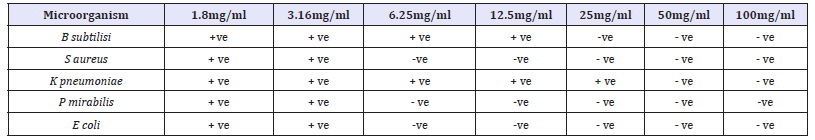
Key: +ve = Growth was observed, -ve = No growth observed
Table 7 shows the minimum inhibitory concentration (MIC) value of Anogeissus leiocarpus against the test organisms used in the study. From this table, C. albicans, A. flavus and Yeast were the fungi with the least MIC values, having their MIC values at 6.25mg/ml respectively, while Aspergillus Niger and F. solani were the test fungi with the highest MIC value, having its MIC value at 12.5mg/ml. it was concluded that at the MIC values of 6.25mg/ml and 12.5mg/ml all the test organism will be inhibited. Table 7 shows the MBC value of the Nauclea latifolia against the test clinical bacteria. From the table, K. pneumoniae and E-coli were observed to be the bacteria with the least MBC values, having their MBC values at 25mg/ml, while B. subtilis, and S. aureus are the test bacteria with the highest MBC values, having MBC values at 100mg/ml respectively.
Table 8 shows the MFC value of the Nauclea latifolia against the test organisms. From this table, F. solani and Yeast were observed to be the fungi with the least MFC values, having their MFC values at 25mg/ml, while A. niger, C. albicans and A. flavus are the test fungi with the highest MFC values, having their MFC values at 100mg/ ml respectively. Table 9 showed the qualitative phytochemical analysis of Anogeissus leiocarpu and Nauclea latifolia leaves using methanol and ethyl acetate. Alkaloid,Steroids, Phenol and Tannins were all present while saponin and cardiac glycoside were not found in Anogeissus leiocarpus. Anthraquinone and Flavonoid were also absent in Nauclea latifolia. Table 7 shows the quantitative phytochemical analysis of Anogeissus leiocarpus and Nauclea latifolia leaves using methanol and ethyl acetate. As observed from the analysis of the leave using methanol, phytate and Alkaloid were the two most abundant compounds at varying amount in the extracts.
Table 8:Minimum inhibitory concentration of ethanol Anogeissus leiocarpus leaf extract against selected fungal isolates.
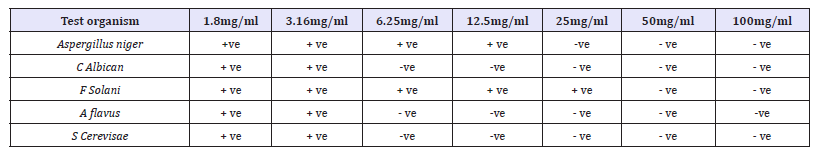
Key: +ve = Growth was observed, -ve = No growth observed
Table 9:Minimum bactericidal concentration of ethanolic extract Nauclea latifolia leaf.
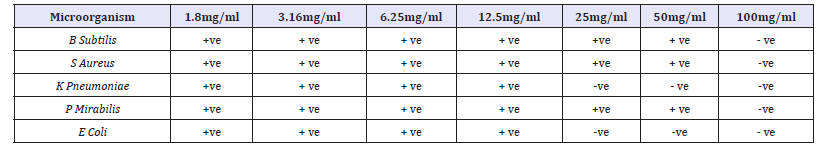
Key: +ve = Growth was observed, -ve = No growth observed
Discussion
The purpose of this work was to investigate the synergistic activity of activated carbon with medicinal plant, in-vitro antibacterial and antifungal properties of Activated carbon (Carcoal), Nauclea latifolia and Anogeissus leiocarpus extract, using ethanol and ethyl acetate as the extracting solvents on selected clinical isolates. In this study, two different plant leaves of Nauclea latifolia and Anogeissus leiocarpus and activated carbon were tested for their antimicrobial properties against selected clinical isolates. The bacteria used are Salmonella typhi, Staphylococcus aureus, Klebsiella pneumoniae, Proteus mirabilis, Escherichia coli, Staphylococcus aureus and Bacillus subtilis and fungi used are Fusarium solani, Aspergillus Niger, Aspergillus flavus, Candida albican and Yeast.
The crude plants extract and activated carbon tested in this study showed antimicrobial activities against all the test bacterial isolates. However, differences were observed between their antibacterial activities. These differences could be attributed to the differences in their chemical composition and amount of the bioactive compounds extracted by the solvent. These compounds usually accumulate in different parts of the plants and such secondary metabolites have been found to produce many effects including antibacterial and antifungal activities [15]. In line with the observation of [16] who reported that that different compounds possessed by different plant is responsible for the antimicrobial activity. The zones of inhibition for the ethanol extract of Anogeissus leiocarpus leaf range from 25mm-17mm at a concentration of 100mg/ml, and 15mm-30mm at 12.5mg/ml. Escherichia coli was discovered from this study to be the most susceptible organism to the ethanol extract of Anogeissus leiocarpus, while Klebsiella pneumoniae was seen to be the least susceptible to this extract. Other test organisms showed very good susceptibility to the leaf extract, as observed from Anogeissus leiocarpus.
The information from this study, can be a good source of a novel antimicrobial agent, especially with good activities against organisms like Escherichia coli and K. pneumoniae, which are resistant to the antibiotic Levofloxacin (positive control), and are seen to be susceptible to this extract. This implies that the leaf of the Anogeissus leiocarpus possesses some active phytochemicals that can inhibit the growth of some microorganisms [17]. The synergetic effect of activated carbon and Anogeissus leiocarpus leaf were observed to be a very active agent against some of the bacteria isolates, with P. mirabilis being the most susceptible bacteria isolate among the tested isolates, having 25.00mm zone of inhibition at 100mg/ml and 15mm zone of inhibition at 12.5mg/ml and Staphylococcus aureus having the least susceptibility of 20mm zone of inhibition at 100mg/ml concentration. The most glaring aspect is the antibacterial activity of Anogeissus leiocarpus extract of leaf having a relatively wide antibacterial activity against P. mirabilis at a concentration of 15mg/ml, a bacterial isolate that was resistant to tetracycline. The ethanol leaf extract also exhibited moderate inhibitory activities against K. pneumonaie.
There is no data yet on the synergistic effect of activated carbon (Charcoal) and medicinal plant. this study can be regarded to be novel in account of the observable data collected during the course of the research work. The synergistic effect of activated carbon and Anogeissus leiocarpus leaf as reported in (Table 2). Activated Carbon (charcoal), Anogeissus leiocarpus and Nauclea latifolia was observed also to be very active against some fungi isolate in this study, with A. flavus being the most susceptible fungi isolate among the tested isolates, having 24.00mm zone of inhibition at 100mg/ ml and 15mm zone of inhibition at 12.5mg/ml and Aspergillus Niger having the least susceptibility of 20mm zone of inhibition at 100mg/ml concentration. The most striking features and glaring aspect was the antifungal activity of Anogeissus leiocarpus extract of leaf having relatively good antifungal activity against C. albican at a concentration of 21mg/ml, a fungal isolate that was found to be resistant against tetracycline. The ethanol leaf extract also exhibited very promising inhibitory activities against F. solani. Despite electrostatic repulsion between negatively charged microorganisms and carbon surfaces, microorganisms attach to activated carbon particles through a strong mechanism called Lifshitz vander Waals forces. Once there is a charge reversal, the electrostatic attraction between negatively charged microbial cell surfaces and positively modified carbon particles will be strong [1].
In the synergistic antimicrobial activity of activated carbon and Nauclea latifolia leaf extract, E-coli was observed to be the most susceptible isolates to the extracts, having zones of inhibition to be 25mm and 10mm at 100mg/ml and 6.25mg/ml respectively. K. pneumoniae was the least susceptible with 15mm and 3mm at 100mg/ml and 6.25mg/ml respectively. Although, it was observed that, the leaf has more inhibitory effect than the activated carbon on some organisms, but on a larger term, with its effect on all the test isolates, Anogeissus leiocarpus leaf was observed to be very potent, especially due to its activity on P. aeruginosa and K. pneumoniae which are resistant to Levofloxacin The results corroborates the findings of [18]. The minimum inhibitory concentration (MIC) value and minimum bactericidal concentration (MBC) value of Nauclea latifolia and Anogeissus leiocarpus was shown in Table 4 and it was shown that the ethanol extract of A. latifolia showed different MIC values against the test isolates , S. aureus, P. mirabilis and E-coli were shown to have the least MBC values with their growth inhibited at 6.25mg/ml respectively, while K. pneumoniae was the bacterial isolate with the highest MIC value, having its growth inhibited at 50mg/ml. B. subtilisi also have relatively high MIC value at 25mg/ml The low MIC of the extract is of great importance to the health care system since it can be used as an alternative to orthodox antibiotics in the treatment of infections due fact that clinical isolates are becoming resistant to known antibiotics [19,20].
The qualitative phytochemical screening of Anogeissus leiocarpus and Nauclea latifolia leaf revealed the presence of medicinally active constituent such as Alkaloid, Tannins, Saponin, reducing sugars, Phenol and Anthraquinone for Anogeisssus leiocarpus leaf (Table 8) above, while for N. latifolia leaf revealed various active constituents such as cardiac glycoside,Steroids, Phenol, Tannins, Saponin some of which have been previously associated with antibacterial activity as observed by Nweze et al. [17]. These biologically active constituent is known to act by different mechanism and exert antimicrobial action. Alkaloids are medicinally useful, possessing analgesic, antispasmodic and bactericidal effects. Flavonoids are hydroxylated Phenolic substance known to be synthesized by plants in response to microbial infection and it should not be surprising that they have been found in vitro to be effective antimicrobial substances against a wide array of microorganisms. Their activity is probably due to their ability to complex with extracellular and soluble proteins and to complex with bacterial cell walls. The antimicrobial property of Saponin is due to its ability to cause leakage of proteins and certain enzymes from the cell [6].Steroids have been reported to have antibacterial properties the correlation between membrane lipids and sensitivity for Steroidal compound indicates the mechanism in whichSteroids specifically associate with membrane lipid and exerts its action by causing leakages from liposomes [21].
The quantitative phytochemical screening of Anogeissus leiocarpus and Nauclea latifolia, leaf using methanol solvent, showed the presence of different phytoconstituents in different quantities. For Anogeissus leiocarpus and Nauclea latifolia using methanol, Alkaloid was shown to be present in the largest quantity in both plant with 9.08 and 10.43, Flavonoid and phytate was found to be the least abundantly present with 2.71 and 1.70 respectively (Table 10). The quantitative phytochemical screening of Anogeissus leiocarpus and Nauclea latifolia leaf using ethyl acetate solvents, showed the presence of different phytoconstituents in different quantities. For Anogeissus leiocarpus and Nauclea latifolia using methanol, Alkaloid was shown to be present in the largest quantity in both plant with 9.08 and 10.43, Flavonoid and Phytate was found to be the least abundantly present with 2.71 and 1.70 respectively Table 11.
Table 10:Minimum fungicidal concentration of ethanolic Nauclea latifolia leaf extract against fungal isolates.

Key: +ve = Growth was observed, -ve = No growth observed
Table 11:Qualitative Phytochemical analysis of Ethanolic and Ethyl acetate extracts of Anogeissus leiocarpus leaf.
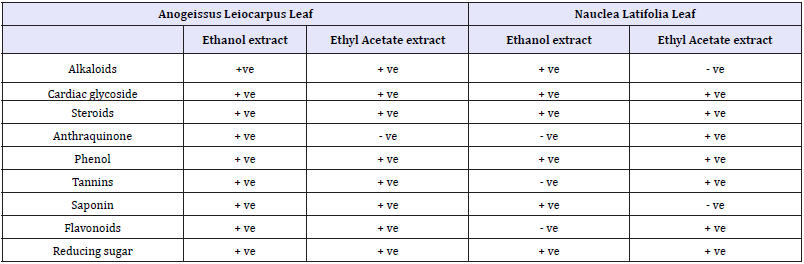
Key: +ve = Present, -ve = Absent
Conclusion
It is very important to know that this study has identified the coagulating activity of activated carbon (Charcoal)and the destruction of the clinical isolates by the two medicinal plants (Anogeissus leiocarpus and Nauclea latifolia), which open a new trend in the synergistic activity of medicinal plant and activated carbon, for the first time, which is a novel discovery (Table 12). The findings of this study have validated that activated carbon has no antibacterial activity rather it coagulates the test isolates but the two medicinal plant i.e., Anogeissus leiocarpus and Nauclea latifolia destroys the microorganisms. Identifying the active pharmacological components of Anogeissus leiocarpus and Nuclea latifolia responsible for antibacterial activities and its mechanisms of action may be the focus of further research activities, while the synergistic effect of activated carbon, Anogeisssus leiocarpus and Nuclea latifolia is of great importance because of its broad spectrum of antimicrobial activities.
Table 12:Quantitative Phytochemical analysis of Ethanolic and Ethyl acetate extracts of Anogeissus leiocarpus leaf.
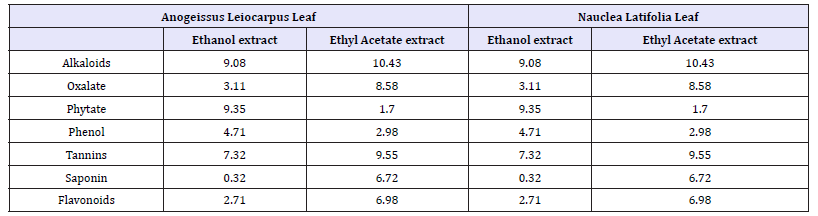
References
- Shi X, Kachirskaia I, Walter KL, Kuo JH, Lake A, et al. (2007) Proteomewide analysis in Saccharomyces cerevisiae identifies several PHD fingers as novel direct and selective binding modules of histone H3 methylated at either lysine 4 orlysine 36. J Biol Chem 282(4): 2450-2455.
- Hsiao FH, Klimidis S, Minas H, Tan ES (2006) Cultural attribution of mental health suffering in Chinese societies: the views of Chinese patients with mental illness and their caregivers. Journal of Clinical Nursing 15(8): 998-1006.
- Priester JH, Stoimenov, Mielke R, Webb S, Ehrhardt C, et al. (2009) Effects of soluble cadmium salts versus Cd Se quantum dots on the growth of planktonic Pseudomonas aeruginosa. Environ Sci Technol 43(7): 2589- 2594.
- Kamboj VP (2000) Herbal medicine. Current Science Association 78(1): 35-39.
- Salau AK, Yakubu MT, Oladiji AT (2013) Cytotoxic activity of aqueous extracts of Anogeissus leiocarpus and Terminalia avicennioides root barks against Ehrlich ascites carcinoma cells. Indian J Pharmacol 45(4): 381- 385.
- Shuaibu MN (2008) Trypanocidal activity of extracts and compounds from the stem bark of Anogeissus leiocarpus and Terminalia avicennoides. Parasitol Res 102(4): 697-703.
- Okwori AEJ, Okeke LI, Uzoechina A, Etukudoh NS, Amali MN, et al. (2008) The antibacterial potentials of Nauclea latifolia. Afr J Biotechnol 7(10): 1394-1399.
- Njume C, Goduka NI (2012) Treatment of diarrhoea in rural African communities: an overview of measures to maximize the medicinal potentials of indigenous plants. Int J Environ Res Public Health 9(11): 3911-3933.
- Osuntokun OT, Olajubu FA (2015) Antibacterial and phytochemical properties of some Nigerian medicinal plant on Salmonella paratyphi isolated from infected human stool in Owo local government. Journal of scientific research and reports 4(5): 441-449.
- Osuntokun OT (2015) Comparative study of antibacterial and phytochemical properties of Nigerian Medicinal Plants on Salmonella bongori and Salmonella enteritidis isolated from poultry feaces in Owo Local Government. Ondo State, Nigeria. Archives of current research international 2(1): 1-11.
- Mallikharjuna PB, Rajanna LN, Seetharam YN, Sharanabasappa GK (2007) Phytochemical studies of Strychnos potatorum L.f.- A medicinal plant. E-J Chem 4(4): 510-518.
- Kumar GS, Jayaveera KN, Kumar CKA, Sanjay UP, Swamy BMV, et al. (2007). Antimicrobial effects of Indian medicinal plants against acneinducing bacteria. Trop J pharm res 6(2): 717-723.
- Parekh J, Chanda SV (2007) In vitro antimicrobial activity and phytochemical analysis of some Indian medicinal plants. Turk Journal Biol 31: 53-58.
- Harborne JB (2005) Phytochemical Methods: A Guide to Modern Techniques of Plant Analysis. (3rd edn), Springer Pvt. Ltd., New Delhi, India, ISBN-13: 9788181283108, pp: 145-156.
- Afolayan AJ (2003) Extracts from the shoots of Arctotis arctoides inhibits the growth of fungi and fungi. Journal Pharm Biol 41(1): 22-25.
- Osadebe PO (2004) Determination and correlation of the reversedphase thin-layer chromatographic parameter (Rm) of a series of 3-(4-substituted-l-piperazinyl)-l-substituted-l-phenyl propanol derivatives with their LD50 values. Boll Chim Farma 143(6): 253-260.
- Nweze EI, Okafor JI, Njoku O (2004) Antimicrobial activities of methanolic extracts of Trema guineensis (Schumm and Thorn) and Morinda lucida Benth used in Nigerian. Bio-Research 2(1): 39-46.
- Zumbes HJ, Belenu TO, Onwuliri FC (2007) In vitro antibacterial activity of Anogeissus leiocarpus leaf extracts on some bacteria associated with diarrhea. International. Journal Natl Applies Sci 3(1): 53-56.
- Shemy HAE, Enein AMA, Enein KMA, Fujita K (2007) Willow leaves extracts contain anti-tumor agents effective against three cell types. Plos One 2(1): e178.
- Prasannabalaji N, Muralitharan C, Sivanandan RN, Kumaran S, Pugazhvendan SR (2012) Antifungall activity of some Indian traditional plants extracts. Asian Pac Journal Trop Dis 2(Supplement 1): S291-S295.
- Raquel FE, Savage PB, Epand RM (2007) Bacterial lipid composition and the antimicrobial efficacy of cationic steroid compounds. Biochemical Biophysic Acta 1768(10): 2500-2509.
© 2018 Oludare Temitope Osuntokun. This is an open access article distributed under the terms of the Creative Commons Attribution License , which permits unrestricted use, distribution, and build upon your work non-commercially.
 a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in
a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in 







.jpg)






























 Editorial Board Registrations
Editorial Board Registrations Submit your Article
Submit your Article Refer a Friend
Refer a Friend Advertise With Us
Advertise With Us
.jpg)






.jpg)














.bmp)
.jpg)
.png)
.jpg)










.jpg)






.png)

.png)



.png)






