- Submissions

Full Text
Modern Applications in Pharmacy & Pharmacology
Antibacterial Activity of Fractionated Extracts of Carica papaya Leaves and Stem Bark against Clinical Isolates of Methicillin Resistant Staphylococcus aureus (MRSA)
Auwal Umar1, Olanitola SO2, Lawan Fagwalawa D3 and Muhammad Ali4*
1Department of Biological Science Unit, Ahmadu Bello University, School of Basic and Remedial studies, Nigeria
2Department of Microbiology, Ahmadu Bello University, Nigeria
3Department of Biology, Kano University of Science and Technology, Nigeria
4Department of Microbiology, Kano University of Science and Technology, Nigeria
*Corresponding author: Muhammad Ali, Department of Microbiology, Kano University of Science and Technology Wudil, Nigeria
Submission: March 8, 2018; Published: March 22, 2018

ISSN 2637-7756Volume1 Issue5
Abstract
The research was aimed to determine the phytochemical screening and antibacterial activity of fractionated leaves and stem bark extracts of Carica papaya on six different clinical isolates of Methicillin Resistant Staphylococcus aureus (MRSA) recovered from infected patients attending Murtala Muhammad Specialist Hospital. Preliminary phytochemical screening of the extracts showed the presence of alkaloids, saponin, phenol, flavonoids, Protein and amino acid, reducing sugar, anthraquinone steroid and terpenoid. Using agar well diffusion method for determination of antibacterial activity of the extracts, the results showed that the fractionated leaves extracts showed higher activity against the isolates (with average zone of inhibition of 16.40±1.55 mm) with n-hexane fraction showing higher antibacterial activity (18.23±1.12 mm) than ethyl-acetate (15.35±1.04 mm) and n-butanol (13.33±0.95 mm) fractions while the isolates were resistant to stem bark extract. Statistical analysis of the results showed that ISOL. 3 is the most susceptible to the extracts used covering an average zone of inhibition of 19.83 ±1.10 mm followed by ISOL 6 (19.66±1.06 mm). Least zone of inhibition was recorded in ISOL 5 (09.80±0.31 mm) which is resistant to the extracts used. There is statistical significant different on the activity of the extracts and susceptibility of the organisms against the extracts at p<0.05. Based on the findings of this research, the ethno botanical application of the plant (Carica papaya) is justified.
Keywords: Antibacterial activity; Carica papaya; Phytochemical; Methicillin resistant Staphylococcus aureus
Abbreviations: DMSO: Dimethylsulphuroxide; MRSA: Methicillin Resistant Staphylococcus aureus; TLC: Thin Layer Chromatography
Introduction
Since ancient times, herbs and their essential oils have been known for their varying degrees of antimicrobial activity [1]. More recently, medicinal plant extracts were developed and proposed for use in food as natural antimicrobials [2-4]. In recent times, research interest for active chemical agents against MRSA, especially from indigenous medicinal plants resources has received the attention of pharmacists across the globe [5-7]. However, little or no work has been done on the effects of papaya extracts on methicillin resistant Staphylococcus aureus in Northern Nigeria.
The importance of herbs in the management of human ailments cannot be over emphasized. It is clear that the plant kingdom harbors an inexhaustible source of active ingredients invaluable in the management of many intractable diseases. Furthermore, the active components of herbal remedies have the advantage of being combined with other substances that appear to be inactive. However, these complementary components give the plant as a whole a safety and efficiency much superior to that of its isolated and pure active components [8]. There is no plant that does not have medicinal value. The active components are normally extracted from all plant structures, but the concentrations of these components vary from structure to structure. However, parts known to contain the highest concentration of the principles are preferred to therapeutic purposes and it can either be the leaves, stems, barks, roots, bulks, corms, rhizomes, woods, flowers, fruits or the seeds [9].
Carica papaya L. belongs to the family of Caricaceae, and several species of Caricaceae have been used as a remedy against a variety of diseases [10]. Carica papaya L. is commonly called pawpaw (English), Gwanda (Hausa), Ibebe (Yoruba) or Okoegbe (Igbo) [11]. It is a mono-sexual plant of Central American origin [11]. Besides the fruit been edible, it has been reported that the roots and the leaves have been used as antihelminthes and for the treatment of infections of bacterial origin [12]. Papaya leave extracts have phenolic compound and caffeic acid [13]. Carica Papaya plant produce natural compound in leaf and bark as well as twig tissues that poses both highly anti-tumor and pesticide properties [14]. The aim of the study is to determine the antibacterial activity and phytochemical constituents of fractionated leaves and stem bark extract of Carica papaya against clinical isolates of Methicillin Resistant Staphylococcus aureus MRSA.
Materials and Methods
Ethical approval
Ethical approval (issue number HMB/GEN/488/VOL. 1) was obtained from the Murtala Mohammed Specialists Hospital (MMSH), Kano based on the consent of the Hospitals Ethical Committees.
Experimental microorganisms
The experimental organisms (six different isolates of Methicillin Resistant Staphylococcus aureus) were isolated from clinical samples of high virginal swab, wounds and skin of patients from hospital patients presenting symptoms of Staphylococcus aureus -associated diseases attending Murtala Mohammed Specialists Hospital Kano, Nigeria. The isolates were identified by standard method [15]. The pure culture of the confirmed isolates were preserved on Nutrient agar slants, labeled, transported to Microbiology Laboratory of Kano State University of Science and Technology Wudil and stored in refrigerator at 4 0C for subsequent tests.
Collection of plant materials
Fresh green leaves and stem bark of Carica papaya were collected from Biological garden of Ahmadu Bello University School of Basic and Remedial Studies Funtua. The plant materials were carried to Herbarium in the Department of Biological Sciences, Ahmadu Bello University Zaria, Nigeria where they were authenticated. A voucher number was issued as 307. The plant materials were washed thoroughly 5 times in sterile distilled water. Then air-dried under shade at room temperature for 14 days and pulverized to finely powdered form using pestle and mortar as described by Ali et al. [16].
Preparation of extracts
The crude extract from leaves and stem bark of Carica papaya was prepared according to the method proposed by Alabi et al. [17]. Fifty grams of powdered sample of plant's parts were extracted exhaustively (cold maceration) with distilled water and ethanol for 7 seven days. The extracts were filtered using Whatman No. 2 filter paper, and concentrated at reduced pressure in water bath (for aqueous extract) and rotary evaporator (for ethanol extract) at 40 °C to afford the various crude extracts of the plant' s parts. The samples were kept in the refrigerator at 4 °C until use.
Fractionation of the crude extract
The partitioning procedure was done using solvents of three different polarities. 80g of the Crude extract was dissolved in 600ml distilled water in a measuring cylinder, shaken and allowed to stand for 10 minutes. The mixture was transferred in to separation funnel followed by addition of normal hexane, the mixture were allowed to stand for the some 15 minutes for partitioning to take place. The tap from the separation funnel was released and the fraction collected inside a sterile bottle. The same procedure was repeated for normal n-butanol and ethyl acetate. Each fraction was transferred into evaporation dish and evaporated using water bath at 45 °C. After evaporation each fraction was weighed, recorded and transferred into sterile bottle for sensitivity test. A concentration of 400mg/ml was prepared for each of the fraction by weighing 4g of the plant extract in 10ml of 10% DMSO as stock solution.
Thin Layer Chromatography (TLC) of the fractions
Thin layer Chromatography was done using TLC prepared plate as described by Sherma & Fried [18]. Small amount of the fractions was dissolved in distilled water in a separate test tube. Each fraction that of the extract, Hexane, Ethyl-acetate, butanol and aqueous fraction were spotted on TLC prepared plate using capillary tube. The TLC plates was then placed inside TLC tank containing two different solvent system, hexane ethyl acetate 3:7 and butanol Acetic acid: water 3:1:1. After 30 minutes, the TLC plates were removed and allowed to dryness, it then sprayed with detecting agent of 0.5ml of para-anisaldehide in 50ml of glacial acetic acid and 1ml of 97%. Concentrated Tetraoxosulphate (VI) acid (H2SO4) and heated inside ovum at 105 °C. The movement of the spots was viewed and their respective Retention Factor (Rf) values were recorded.
Phytochemical Screening
The extracts were subjected to various phytochemical tests to identify the phytochemical constituents present using standard methods as described by Sofowora [19], Trease & Evans [20]. Phytochemical screening was performed to test for alkaloids, saponin, phenol, flavonoids, Protein and amino acid, reducing sugar, tannin, anthraquinone steroid and terpenoid.
Determination of antibacterial activity of the extracts
Antibacterial activity of the aqueous and ethanolic n- hexane, n- butanol and ethyl acetate fractionated extracts of the leaf and stem bark of Carica papaya were determined using agar well diffusion method as described by Aida et al. [21]. For the test, Muller- Hinton agar plates were swabbed with standard test isolates (0.5 McFarland), two wells were made on the surface of the agar using 6mm sterile steel borer and the wells (6mm) were filled with 400mmg/ml concentration of 0.1ml of each extract and 200mg/ml of Tetracycline as a positive control. The cultures were incubated at 37 °C for 24 hours. The antibacterial potential of test extracts was determined on the basis of diameter of zone of inhibition around the wells as described by Sumitra & Sharma [22].
Statistical Analysis
The zone of inhibition produced by the isolates against the extracts used was analyzed using One-Way ANOVAs using statistical program SPSS 21.0 (Statistical Package for the Social Sciences). Significance level for the differences was set at p<0.05.
Results
Sources of the isolates
Six (6) different isolates of Methicillin Resistant Staphylococcus aureus) were isolated from clinical samples of high virginal swab (n=1), wounds (n=3) and skin (n=2) of patients from hospital patients presenting symptoms of Staphylococcus aureus-associated diseases attending Murtala Mohammed Specialists Hospital Kano, Nigeria (Table 1).
Table 1: Various sources of the isolates used.
Phytochemical screening
Table 2: Phytochemical constituents of leaves and stem of Carica papaya.

The phytochemical screening of the active phytochemical constituents of the extracts is presented in Table 2. Leaf extract contain alkaloids, saponin, phenol, flavonoids, Protein and amino acid, reducing sugar, anthraquinone steroid and terpenoid except Tannin, while stem extract contain only three phytochemicals Alkaloid, Saponin and Flavonoids. The result showed that Carica papaya leaves extract contain more phytochemicals than the stem bark extract.
Antibacterial activity of fractionated leaves extracts
The antibacterial activity of the fractionated plant leaf extract (at 400mg/ml) against the 6 isolates of MRSA is presented in Table 3. The result shows that highest zone of inhibition was shown by n-hexane extract with zone of inhibition of 22mm. lowest zone of inhibition was recorded in n-butanol (8mm). Isolate 3 and 6 were found to be more sensitive to the extract while isolate 5 was more resistant.
Table 3: Antibacterial activity of the fractionated leaf extracts.
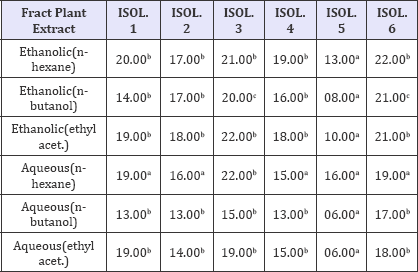
Key: 06.00 = No zone of inhibition, Values having different superscript on the same row are considered significantly different at p<0.05
Antibacterial activity of fractionated stem bark extracts
The antibacterial activity of the fractionated stem bark extract (at 400mg/ml) against the 6 isolates of MRSA is presented in Table 4. The result shows that highest zone of inhibition was shown by acetyl acetate extract with zone of inhibition of 11mm. most of the isolates were found to be resistant to the various fractions of the stem extract
Table 4: Antibacterial activity of the fractionated stem extracts.
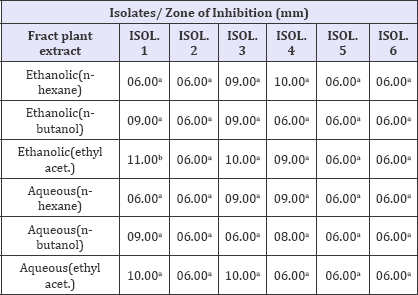
Key: 06.00 = No zone of inhibition, Values having different superscript on the same row are considered significantly different at p<0.05
Discussion
The result for phytochemical screening of Carica papaya showed that the plants contained some phytochemical compounds which possess good antimicrobial properties on the test clinical isolates used in the study. The phytochemical analysis of the plant showed that the leaves contain Anthraquinones, phenols, glycoside, amino-acid, terpenoid, reducing sugar, Saponin, Tannin, Alkaloids and Flavonoids. On the other hand, the stem extract contains Alkaloid, saponin, flavonoids and reducing sugar. This finding can be attested to the work of Sikanda et al. [23] who also reported similar finding and also stated the effect of these phytochemical as a good antimicrobial agent on different test organism. Doughari et al. [11] reported the anti-bacterial effect of the extract of C. papaya on various bacterial isolates including Staphylococcus aureus, Salmonella typhi and Bacillus cereus. Although the mechanism of action of this extract is not understood, it has been proposed that its action against the bacteria and fungi may be due to the inhibition of cell wall formation in the cell resulting in a leakage of cytoplasmic constituents by the bioactive components of the extract [24,25].
In addition, bioactive substances have been reported to confer resistance to plants against bacteria, fungi and pests and therefore explain the demonstration of antibacterial activity by the plant extracts used in this study [26]. In these regard, Aravind et al. [27] reported that the many benefits of papaya, are due to the high content of Vitamins A, B and C, proteolytic enzymes like papain and chymopapain that have antiviral, antifungal and antibacterial properties while phytochemical compounds such as tannin coagulate the wall proteins, saponins facilitated the entry of toxic material or leakage of vital constituents from the cell [28].
Flavonoids inhibit the activity of enzymes by forming complexes with bacterial cell walls, extracellular and soluble proteins, more lipophilic flavonoids disrupt cell wall integrity [29] or microbial membranes [30] at low concentrations. The existence of Saponin supports the fact that pawpaw has cytotoxic effect such as permealization of the intestine as Saponins are cytotoxic [31]. Alkaloids are the most efficient therapeutically influential plant substance. Pure natural and synthetic derivatives of alkaloids are used as a basic medical agent because of their analgesic, antispasmodic and antibacterial properties [32].
The presence of Alkaloid in the pawpaw shows that this plant can be an effective anti-malaria agent since alkaloid consists of quinine, which is anti-malaria [33]. Marchese & Shito [34], Poole [35] reported the sensitivity of the microbial strains to both the plant extracts and to synthetic antibiotics, and observed that that the plant extracts compete favorably with the drugs and can be used as an alternative to the antibiotics as the zones of inhibition shown were very comparable and the extracts have lesser side effects which are often associated with the use of antibiotics. Also, the issue of resistance to these extracts cannot arise as is found with antibiotics [36].
The present study showed that the leaves of Bacillus cereus papaya possess antimicrobial potential against MRSA. In line with the present finding, several other studies [37-39] have reported Cereus papaya leaves have antimicrobial potentials and have significant antibacterial activity in various extracts from different tree's parts. The antimicrobial activity of fractionated extracts of Carica papaya leaves showed zone of inhibition against the isolates tested. The extract of n-hexane fraction exhibited highest zone of inhibition with average of 18.23mm at 400mg/ml while n-butanol and ethyl-acetate fraction has an average of 13.33 and 15.60mm at 400mg/ml respectively. The result of his study supported that of Yahaya et al. [40] who the aqueous and ethanol extract of Carica papaya leaves active against Escherichia coli, S. typhi, Pseudomonas aeruginosa and Staphylococcus aureus.
Conclusion
According to this study, ethanol extracts demonstrated a higher activity than the aqueous extracts in both extracts. The better efficacy of the ethanol extract as against the aqueous extract may be because different solvents have different polarities, hence different degrees of solubility for the various phytoconstituents. It was also found that leaves extracts of the plant possessed higher antibacterial efficacy when compared to stem bark extracts. Phytochemical screening of the extracts showed the presence of anthraquinone, phenols, glycoside, and amino-acid, terpenoid, reducing sugar, Saponin, alkaloids and flavonoids. The phytochemicals are responsible for the antibacterial activity of the extracts.
Acknowledgement
The authors wish to acknowledge the staff of Microbiology Department of Murtala Muhammad Specialist hospital Kano for isolates provision. Thanks to Microbiology Department, Kano University of Science and Technology Wudil, Kano for use of Laboratory facilities.
References
- Chang HW (1995) Antibacterial effect of spices and vegetables. Food Industries 27: 53-61.
- Del Campo J, Amiot MJ, Nguyen The C (2000) Antimicrobial effect of rosemary extract. J Food Protection 63(10): 1359-1368.
- Hsieh PC (2000) Antimicrobial effect of cinnamon extract. Taiwanese Journal of Agricultural Chemistry and Food Science, 38(2): 184-193.
- Hsieh PC, Mau JL, John SH (2001) Antimicrobial effect of various combinations of plant extracts. Food Microbiol 18(1): 35-43.
- Polombo EA, Semple SJ (2002) Antibacterial activity of Australian plants extracts against Methicillin-resistant Staphylococcus aureus (MRSA) and Vancomycin-Reisitant Enterococci (VRE). J Basic Microbiol 42(6): 444-448.
- Voravuthikunchai SP, Kitpipit L (2005) Activity of medicinal plant extracts against hospital isolates of methicillin resistant Staphylococcus aureus. Clin Microbiol Infect 11(6): 510-512.
- Aqil E, Zunita Z, Hassan L, Chen HC (2010) Phenotypic and genotypic characterization of methicillin-resistant Staphylococcus aureus (MRSA) isolated from dogs and cats at university veterinary hospital, Universiti Putra Malaysia. Trop Biomed 27(3): 483-492.
- Ahmad I, Beg AZ (2001) Antimicrobial and Phytochemical studies on 45 Indian medicinal plants against multi-drug resistant human pathogens. J Ethnopharmacol 74(2): 113-123.
- Kafaru E (1994) Immense help from nature's workshop. Elika Health Services Ltd, Academic Press Plc, Nigeria, pp. 1-27.
- Mello VJ, Gomes MT, Lemos FO, Delfino JL, Andrade SP, et al. (2008) The gastric ulcer protective and healing role of cysteine proteinases from Carica candamarcensis. Phytomedicine 15(4): 237-244.
- Doughari JH, El Mahmud AM, Manzara S (2007) Studies on the antibacterial activities of root extract of Carica papaya L. Afr J Microbiol Res, pp. 37-41.
- Fajimi AK, Taiwo AA, Ayodeji H, Adebowale EA, Ogundola FI (2001) Therapeutic trials on gastrointestinal helminthes parasites of goat busing pawpaw seeds as a drench. International Institute of Tropical Agriculture (IITA), Nigeria.
- Ayoola PB, Adeyeye A (2010) Phytochemical and nutrient evaluation of Carica papaya (pawpaw) leaves. IJRRAS 5(3): 325-328.
- Nirosha N, Mangalanayaki R (2013) Antibacterial Activity of Leaves and Stem Extract of Carica papaya L. International journal of advances in pharmacy, biology and chemistry 2(3): 473-476.
- Holt JG, Krieg NR, Sneath PA, Stanley JT, Williams ST (1994) Bergey's manual of systematic bacteriology. (9th edn), Williams & Wilkins Co. Baltimore, USA, p. 786.
- Ali M, Yahaya A, Zage AU, Yusuf ZM (2017) In vitro Antibacterial Activity and Phytochemical Screening of Psidium guajava on Some Enteric Bacterial Isolates of Public Health Importance. Journal of Advances in Medical and Pharmaceutical Sciences 12(3): 1-7.
- Alabi OA, Haruna MT, Anokwuru CP, Jegede T, Abia H, Okegbe VU and Babatunde EE (2012) Comparative studies on antimicrobial properties of extracts of fresh and dried leaves of Carica papaya (L) on clinical bacterial and fiungal isolates. Advances in Applied Science Research 3(5): 3107-3114.
- Sherma J, Fried B (2003) Handbook of Thin-Layer Chromotography. (3ri edn), CRC Press, USA, pp. 1-1048.
- Sofowora A (1993) Medicinal Plants and Traditional Medicine in Africa. (2nd edn), Spectrum Books Ltd, Nigeria, pp. 289.
- Trease GE, Evans WC (1989) Pharmacognosy. (13th edn), ELBS Oxford University Press, UK, pp. 245-263.
- Portillo A, Vila R, Freixa B, Adzet T, Canigueral S (2001) Paraguyan plants used in traditional medicine. J Ethnopharmacol 76(1): 93-98.
- Sumitra S, Sharma SK (2006) The in vitro antibacterial efficiency of essential oil and root extract of Coriandrum sativum Linn. Journal of Agricultural Biological Research 22: 144-149.
- Sikandar KS, Tasveer ZB, Kanwal N, Syed AG, Shahama UK (2013) Qualitative phytochemical screening and antifungal activity of Carica papaya leaf extract against human and plant pathogenic fungi. Int Res J Pharm 4(7): 83-86.
- Bais HP, Walker TS, Schweizer HP, Vivanco, JM (2002) Root specific elicitation and antimicrobial activity of rosmarinic acid in hairy root cultures of Ocimum basilicum. Plant Physiology and Biochemistry 40(11): 983-995.
- Hassan SW, Umar RA, Ladan MJ, Nyemike P, Wasagu RSU, et al. (2007) Nutritive Value, Phytochemical and Antifungal Properties of Pergularia tomentosa L. (Asclepiadaceae). International Journal of Pharmacology 3(4): 334-340.
- Srinivasan D, Perumalasamy LP, Nathan S, Suresh T (2001) Antimicrobial activity of certain Indian medicinal plants used in folkloric medicine. J Ethnopharmacol 74(3): 217-220.
- Aravind G, Debjit B, Duraivel S, Harish G (2013) Traditional and Medicinal Uses of Carica papaya. Journal of Medicinal Plants Studies 1(1): 7-15.
- Onwuliri FC, Wonang DL (2005) Studies on the combined Antimicrobial action of Ginger (Zingiber officinale L) and Garlic (Allium sativum L) on some Bacteria. Nigeria J Bot 18: 224-228.
- Kurtz MB, Heath IB, Marrinan J, Dreikhorn S, Onishi J, Douglas C (1994) Morphological effects of lipopeptides against Aspergillus fumigatus correlate with activities against (1,3)-beta-D-glucan synthase. Antimicrob Agents Chem 38(7): 1480-1489.
- Tsuchiya H, Sato M, Miyazaki T, Fujiwara S, Tanigaki S, et al. (1996) Comparative study on the antibacterial activity of phytochemical flavanones against methicillin resistant Staphylococcus aureus. J Ethnopharmacol 50(1): 27-34.
- Okwu DE, Okwu ME (2004) Chemical composition of Spondias mombin Linn. Plant parts. J Sustain Agric Environ 6(2): 140-147.
- Stray F (1998) The National Guide to Medicinal Herbs and Plants. Tiger Books International, UK, pp. 12-16.
- Robinson S (1995) Kandungan organic Tumbuhan tinggi diterjemahkan Padnawinata K, Edisike-6 Institute Technology, Indonesia.
- Marchese A, Shito GC (2001) Resistance patterns lower respiratory tract pathogens in Europe. Int J Antimicrob Agents 16(Suppl 1): 25-29.
- Poole K (2001) Multidrug efflux pump and antimicrobial resistance in Pseudomonas and related organisms. J Mol Microbiol Biotechnol 3(2): 255-264.
- Kareem KT, Kareem SO, Adeyemo OJ, Egberongbe RK (2010) In vitro antimicrobial properties of Bridelia ferruginea on some clinical isolates. Agriculture and Biological Journal of North America 1(3): 416-420.
- Baskaran C, Ratha bai V, Velu S, Kumaran K (2012) The efficacy of Carica papaya leaf extract on somebacterial and a fungal strain by well diffusion method. Asian Paicfic Journal of Tropical Disease 2012: S658-S662
- Anibijuwon II, Udeze OA (2009) Antimicrobial Activity of Carica Papaya (Paw-paw Leaf) on Some Pathogenic Organisms of Clinical Origin from South-Western Nigeria. Ethnobotanical Leaflets 2009(7): 1-12.
- Ifesan BOT, Fashakin JF, Ebosele F, Oyerinde SA (2013) Antioxidant and antimicrobial properties of selected plant leaves. European Journal of Medicinal Plants 3(3): 465- 473.
- Yahaya A, Ali M and Idris A (2017) Antibacterial Activity and Phytochemical Screening of Carica papaya on some Enteric Bacterial Isolates of Public Health Importance. Greener Journal of Biological Sciences 7(1): 001-007.
© 2018 Auwal Umar, et al. This is an open access article distributed under the terms of the Creative Commons Attribution License , which permits unrestricted use, distribution, and build upon your work non-commercially.
 a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in
a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in 







.jpg)






























 Editorial Board Registrations
Editorial Board Registrations Submit your Article
Submit your Article Refer a Friend
Refer a Friend Advertise With Us
Advertise With Us
.jpg)






.jpg)














.bmp)
.jpg)
.png)
.jpg)










.jpg)






.png)

.png)



.png)






