- Submissions

Full Text
Modern Applications in Pharmacy & Pharmacology
An Overview of Enalapril by UV Spectropho- tometer and HPLC
Aymen Owais Ghauri, Safila Naveed*, Hina Rehman, Saima Asif and Fatima Qamar
Faculty of Pharmacy, Jinnah University for Women Karachi, Pakistan
*Corresponding author: Safila Naveed, Faculty of Pharmacy, Jinnah University for Women Karachi, Pakistan, 74600
Submission: January 27, 2018; Published: March 22, 2018

ISSN 2637-7756Volume1 Issue5
Abstract
Present review article determine the analytical methods for the quantitative determinations of Enalapril (ACE Inhibitor) by one of the spectroscopic technique (UV spectrophotometry) and separation technique such as High-Performance Liquid chromatography (HPLC).Pharmaceuticals dosage formulations and human serum is needed for effective analytical procedure and quality control of Enalapril in clinical and pharmaceutical practices. An extensive survey of the literature published in various pharmaceutical and analytical chemistry related journals has been compiled in its review. A synopsis of reported spectrophotometer and high-performance liquid chromatographic methods for Enalapril is integrated. This appraisal illustrate that majority of the HPLC methods reviewed are based on the quantitative analysis of drug in API (active Pharmaceutical ingredients) biological fluids and they are appropriate for therapeutic drug monitoring purpose.
Keywords: HPLC; UV spectrophotometer; Quality control analysis; Method development; Validation
Introduction
Angiotensin converting enzyme that cleaves the terminal two peptides from angiotensin I (decapeptide) is blocked by the ACE inhibitors to form the powerful vasoconstrictor angiotensin II (octapeptide) and decreases the BP by reducing peripheral vascular resistance, it nither increases the rate of cardiac output nor contractility. Almost all ACE inhibitors have relatively same antihypertensive ability they efficiently block the angiotensin 1 conversion to angiotensin II and have alike therapeutic indications, contraindications and adverse effect profile.
Enalapril(S)-1-[N-[1-(ethoxycarbonyl)-3-phenylpropyl]-L- alanyl]-L-proline, (Z)-2-butenedioate [1] is the maleate enalapril salt a derivative of 2-amino acid, L-alanine and L-proline. Soluble in Aqueous, MeOH and ethanol preperations. Enalapril maleate comprises of crystalline white to off-white powder which is synthesized chemically; an antihypertensive and a vasodilator in congestive cardiac failure, and for managing hypertension it is used with hydrochlorothiazide [2]. The pro-drug enalapril is biologically activated by hydrolysis of the ethyl ester to active form enalaprilate. Now, enalaprilate after this conversion inhibits angiotensin-converting enzyme (ACE) in mammals. Conversion of angiotensin I is catalyzed by ACE i.e. peptidyl dipeptidase to the vasoconstrictor substance. Angiotensin II works on adrenal cortex and stimulates aldosterone secretion. The primary effects of enalapril in cardiac failure and high blood pressure seems from suppression of the renin-angiotensin-aldosterone system. Reduced plasma angiotensin II results from inhibition of ACE, which leads to reduced vasopressor activity and reduced aldosterone secretion.
Methods of Assay
Enalapril maleate was determined in pharmaceutical tablets using first-derivative ultraviolet spectrophotometry [3-5]. Spectrophotometric and paleographic determination of enalapril and lisinopril using 2, 4-dinitrofluorobenzene has been studied [6]. Isothermal microcalorimetry and HPLC were used to determine the stability of enalapril, EM and other different tablet formulations [7]. The reversed phase high-performance liquid chromatography (RP-HPLC) was implied to view the kinetics of Z-(cis)/E-(trans) isomerization of enalapril [8,9]. For indirect method of enalapril in human plasma [10] a Lichrosphere® (125mm*4.0mm i.d.) column at flow rate of 1.0mLmin-1 was done by high-performance liquid chromatography. The lowest concentration to be quantities was 3.0ngmL-1 with the acceptable accuracy and precision, the time taken was 6.5 minutes and this method was used for bioequivalent and pharmacokinetics study of enalapril. The influence of temperature (from 383 to 348K) and relative humidity (from 25.0 to 76.4%) on the stability of enalapril maleate in the solid phase was investigated and. changes in the concentration of enalapril maleate were followed by a HPLC method with UV detection [11]. The kinetic and thermodynamic parameters [Ea (kJ mol-1) = 168.5±27 for RH=0% and 149.1±48 for RH=76.4%; ΔH* (kJ mol-1) = 166.1±30 for RH=0% and 146.6±50 for RH=76.4%; ΔS* (J (K-1 mol-1)) = 120.3±169 for RH=0% and 82.1±110 for RH=76.4%) of the decomposition reaction were calculated.
Another HPLC method with UV-detection [12] has been developed for the determination of enalaprilate this method produced linear response over the wide concentration range of 1-200μgmL-1. A reversed-phase high-performance liquid chromatographic (RP-HPLC) method was for the simultaneous determination of enalapril and its degradation and felodipine and its degradation product, in the combined enalapril/felodipine (5mg/5mg) formulation [13] using a Spherisorb C8 column with a CH3CN-0.001M KH2PO4 (pH2) (35:65, v/v) mobile phase. Carlucci et al. [4] determined enalapril maleate and hydrochlorothiazide in pharmaceutical formulations by HPLC by utilizing the linear relationship between substances concentration and derivative peak amplitude detectable by derivative spectrophotometry.
Several examinations using HPLC for determination of Enalapril in bulk drug substances and their formulations have been reported [14]. Direct Determination of Four ACE-Inhibitors Lisinopril, Enalapril, Captopril and Fosinopril in Pharmaceuticals and Serum by HPLC, Good separation of the analytes was achieved by gradient RP-HPLC with the mobile phase composed as acetonitrile: water (60:40, v/v) adjusted to pH 3.0 by ortho phosphoric acid [15]. The in vitro interaction studies of enalapril with hypoglycemic agents were monitored by LC-UV [16].
Figure 1: Enalapril.
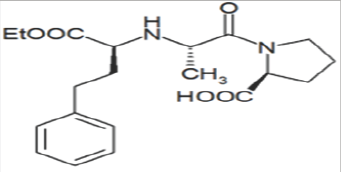
Another Manifest and Facile Liquid Chromatographic Method for the Simultaneous determination of NSAIDs, Enalpril Maleate in API and Pharmaceutical Formulations is reported and ENP was separated from NSAIDs using a Purospher STAR C18 column (250x4.6mm, 5|im) and a mobile phase consisting of methanol, water (80:20v/v, pH was adjusted by ortho phosphoric acid to 2.8 at a flow rate of 1.8mL min-1 and at ambient temperature. Effluents from the column were monitored at 225nm. [17] ACE inhibitors alone are not sufficient to decrease high blood pressure hence, they are used in as combination with other particular classes of drug compounds such as calcium channel blocker antihypertensives, diuretics, and etc another method is reported of enalapril with diuretic Hydrochlorothiazide and Furosemide in Active Pharmaceutical Ingredients, Pharmaceutical Dosage Forms and Human Serum [18]. Simultaneous LC determination of, lisinopril, rosuvastatin, captopril, and enalapril in API, pharmaceutical dosage formulations, and human serum [19] other methods of enalapril with hypoglycemics (glibenclamide .metformin, glimepride) statins and H2 receptor antagonist in bulk, formulations and human serum by RP-HPLC [20-22] are reported (Figure 1).
Conclusion
Patients who are diagnosed with hypertension are prescribed with the large number of medications for appropriate therapy, while, increasing risk of drug interactions and side effects. In this article UV and HPLC methods for the determination of Enalapril in bulk material, pharmaceutical formulations and biological specimens are reviewed alone or in combination with other drugs. Spectrophotometric techniques provided practical and significant economic advantages over other methods; therefore, they are a choice for pharmaceutical analyses. The provision for use and disposal of solvents, expensive equipment, laborintensive sample preparation procedure and personally competent in chromatographic techniques are generally required in HPLC method. Additionally, almost all of the HPLC methods studied has the potential application to multi-drug pharmacokinetics studies, clinical research of drug combination, and interactions studies.
References
- Sweetman SC (2005) Martindale: The complete drug reference. Pharmaceutical Press, UK, pp. 900-901.
- Dominic PI, Gerald SB, Florey K (1987) Analytical profiles of drug substances. Academic Press, USA, pp. 207-243.
- Blaih SM, Abdine HH, El Yazbi FA, Shaalan RA (2000) Spectrophotometric determination of enalapril maleate and ramipril in dosage forms. Spectroscopy Letters 33(1): 91-102.
- Carlucci GD, Giuseppe E, Mazzeo P (1993) Simultaneous determination of enalapril maleate and hydrochlorothiazide in tablets by derivative UV spectrophotometry and high-performance liquid chromatography International Journal of Pharmaceutics 93(1-3): 245-248.
- Elwalily AF, Belal SF, Heaba EA, Elkersh A (1995) Simultaneous determination of enalapril maleate and hydrochlorothiazide by first- derivative ultraviolet spectrophotometry and high-performance liquid chromatography. J Pharm Biomed Anal 13(7): 851-857.
- Abdel Razak O, Belal SF, Bedair MM, Barakat NS, Haggag RS (2003) Spectrophotometric and polarographic determination of enalapril and lisinopril using 2,4-dinitrofluorobenzene. J Pharm Biomed Anal 31(4): 701-711.
- Simon Z, Zupan P, Roskar R, Gartner A, Kogej K, et al. (2007) Use of microcalorimetry in determination of stability of enalapril maleate and enalapril maleate tablet formulations. Int J Pharm 342(1-2): 145-151.
- Shoji A, Yanagida A, Shindo H, Ito Y, Shibusawa Y (2007) Countercurrent chromatographic estimation of hydrophobicity of Z-(cis) and E-(trans) enalapril and kinetics of cis/trans isomerization. Journal of Chromatography A 1157(1-2): 101-107.
- Trabelsi H, Bouabdallah S, Sabbah S, Raouafi F, Bouzouita K (2000) Study of the cis-trans isomerization of enalapril by reversed-phase liquid chromatography. J Chromatogr A 871(1-2): 189-199.
- Thongnopnua P, Poeaknapo C (2005) High-performance liquid chromatographic determination of enalapril in human plasma by enzyme kinetic analytical method. J Pharm Biomed Anal 37(4): 763-769.
- Stanisz B (2003) Evaluation of stability of enalapril maleate in solid phase. J Pharm Biomed Anal 31(2): 375-380.
- Tajerzadeh H, Hamidi M (2001) A simple HPLC method for quantitation of enalaprilate. J Pharm Biomed Anal 24(4): 675-680.
- Qin XZ, De Marco J, Dominic P (1995) Simultaneous determination of enalapril, felodipine and their degradation products in the dosage formulation by reversed-phase high-performance liquid chromatography using a Spherisorb C8 column. Journal of Chromatography A 707(2): 245-254.
- Naveed S, Sultana N, Arayne MS (2012) HPLC-UV method for the determination of enalapril in bulk, pharmaceutical formulations and serum. J Anal Bioanal Techniques 3:130.
- Sultana N, Naveed S, Arayne MS (2013) Direct determination of four ACE-inhibitors lisinopril, enalapril, captopril and fosinopril in pharmaceuticals and serum by HPLC. J Chromat Separation Techniq 4: 179.
- Sultana N, Naveed S, Arayne MS (2013) Monitoring of in vitro interaction studies of enalapril with hypoglycemic agents by LC-UV Research and Reports in Medicinal Chemistry 2013(3): 1-7.
- Sultana N, Naveed S, Arayne MS (2013) Facile and manifest liquid chromatographic method for the simultaneous determination of enalpril maleate and nsaids in api and pharmaceutical formulations. Pharm Anal Acta S2: 1-5.
- Saeed Arayne M, Naveed S, Sultana N (2013) A validated reverse phase liquid chromatographic method for simultaneous analysis of enalapril maleate, hydrochlorothiazide and furosemide in active pharmaceutical ingredients, pharmaceutical dosage forms and human serum. Pharm Anal Acta 4: 244.
- Arayne MS, Sultana N, Tabassum A, Nadir Ali S, Naveed S (2012) Simultaneous LC determination of rosuvastatin, lisinopril, captopril, and enalapril in API, pharmaceutical dosage formulations, and human serum. Medicinal Chemistry Research 21(12): 4542-4548.
- Naveed S, Sultana N, Arayne MS (2011) Simultaneous quantitation of enalapril, metformin, glibenclamide and glimepride in bulk material, pharmaceutical formulations and human serum using RP-HPLC. International Journal Pharma Research Development 3(1).
- Sultana N, Arayne MS, Naveed S (2011) Simultaneous determination of enalapril and statins in pharmaceutical formulations by RP- HPLC. J Chil Chem Soc 56(3): 734-737.
- Sultana N, Arayne MS, Naveed S, Shamshad H (2009) An RP-HPLC method for simultaneous analysis and interaction studies on enalapril maleate and H2-receptor antagonists. Acta Chromatographica 21(4): 547-558.
© 2018 Aymen Owais Ghauri, et al. This is an open access article distributed under the terms of the Creative Commons Attribution License , which permits unrestricted use, distribution, and build upon your work non-commercially.
 a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in
a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in 







.jpg)






























 Editorial Board Registrations
Editorial Board Registrations Submit your Article
Submit your Article Refer a Friend
Refer a Friend Advertise With Us
Advertise With Us
.jpg)






.jpg)














.bmp)
.jpg)
.png)
.jpg)










.jpg)






.png)

.png)



.png)






