- Submissions

Full Text
Modern Applications in Pharmacy & Pharmacology
Flavonoids from Trollius Europaeus Flowers and Evaluation of Their Biological Activity
Ewa Witkowska Banaszczak Orcid*
Department of Pharmacognosy, Poznan University of Medical Sciences, Poland
*Corresponding author: Ewa Witkowska Banaszczak Orcid, Department of Pharmacognosy, Poznan University of Medical Sciences, Swi^cickiego 4, 60-781, Poznan, Poland
Submission: December 14, 2017; Published: February 23, 2018

ISSN 2637-7756Volume1 Issue4
Abstract
Objectives: This paper describes the flavonoid composition of the flowers of Trollius europaeus and the method of isolation thereof as well as provides an attempt at investigating the antioxidant activity of the isolated flavonoids and the anti tyrosinase activity of the extracts from the investigated material.
Methods: The compositional data were acquired by combining results of Nuclear Magnetic Resonance (NMR), Ultraviolet spectroscopy (UV), Electrospray ionisation-tandem mass spectrometry (ESI-MS/MS) analyses and those of an analysis of the products of acid hydrolysis of the compounds. The antioxidant activity of the extracts was studied using the 2,2'-diphenyl-1-picrylhydrazyl (DPPH) assay and the tyrosinase inhibitory activity - with the use of mushroom tyrosinase.
Key finding: Ten flavonoid derivatives of luteolin and apigenin were isolated from the flowers of Trollius europaeus and identified. The investigation into the antioxidant activity revealed that orientin 2”-O-a-arabinopyranoside (4) and orientin 2”-O-p glucopyranoside (5) had a significant antioxidant effect.
Conclusion: The studies conducted led to the development of a method of isolating flavonoid, potentially antioxidant, compounds from T. europaeus. They allowed determining which of the investigated flavonoids demonstrated significant antioxidant activity and could be used as natural antioxidants.
Keywords: Trollius europaeus; Flavonoids; Apigenin; Luteolin; Antioxidants
Introduction
The genus Trollius (Ranunculaceae) comprises 31 species of perennial herbs which grow in the northern hemisphere areas with temperate climates. In Europe and western Siberia the species Trollius europaeus L. is found. The medicinal part is the whole plant. Various parts of the plant have been used for the treatment of scurvy in folk medicine due to its high content of vitamin C [1]. The previous studies into T europaeus leaves, of which I was a coauthor, reported the presence of flavonoids and phenolic acids [2,3]. A more extensive investigation into the flavonoids in T europaeus flowers led to isolation and structural identification of derivatives of luteolin (1-6) and apigenin (7-10). Except for compound 6, which was an O-glycoside, all of them displayed a C-glycoside-like structure. These compounds have been identified and described for the first time for the investigated material, and vitexin 2”-O-p- arabinopyranoside - for the first time in the plant kingdom. Their structures were established on the basis of NMR and MS, UV and comparison with reference samples (standards).
The NMR spectroscopic data included 1H, 13C NMR, COSY, HMQC and HMBC experiments. The antioxidant activity of four of the obtained compounds (4,5,9,10), which had not been investigated before, was subjected to examination with the use of the method with the DPPH radical. This method is based on the reaction of the antioxidant with the oxidant which changes colour from purple to yellow as a result of reduction. The color change was monitored spectrophotometrically by measuring absorbance at the wavelength A=515nm. The antityrosinase activity of the extracts from the T. europaeus flowers was determined for the first time and was measured by spectrometry, as described by Bendaikha [4]. Tyrosinase is responsible for the process of tyrosine hydroxylation to the laevorotatory form of dopamine (L-DOPA) and the oxidative transformation of L-DOPA into L-Dopaquinone, which spontaneously converts into coloured dopachrome. The tyrosinase activity was monitored by dopachrome concentration at 475nm. Dopachrome is not a direct product of the enzymatic reaction; it results from non-enzymatic reactions of cyclisation and oxidisation of the direct product of this reaction-dopaquinone [5]. A number of studies describing the inhibitory activity of flavonoids and extracts containing flavonoids suggest their activity through formation of copper-flavonoid complexes. This activity depends on the quantity and location of hydroxyl groups [6].
Materials and Methods
General experimental procedures
The NMR spectra (1H, 13C NMR) were recorded using a Bruker NMR Avance II 400MHz spectrometer, CD3OD or DMSO with TMS as an internal standard. The ESI-MS mass spectra were measured on a Waters/Micromass (Manchester, UK) ZQ mass spectrometer connected with a UV 996 Waters photodiode detector equipped with an electrospray interface operating in a negative and positive ion mode at an optimized sample cone voltage of 30V. The UV spectra were recorded on a UV/VIS Perkin Elmer Lambda 35 spectrophotometer in MeOH, also after addition of the specific reagents (NaOAc/ H3BO3, AlCl3, AlCl3 /HCl, NaOMe, NaOAc), according to Mabry et al. [7]. The analytical paper chromatography (PC) was carried out on What man paper chromatography 1 CHR, using the solvent system CH3COOH-H2O (P1, 15:85) and EtOAc- HCOOH-H2O (P2, 10:2:3) upper layer); TLC was performed on silica gel 60 plates Merck using EtOAc-HCOOH-CH3COOH-H2O (P3, 100:11:11:26) and n-PrOH-EtOAc-H2O (P4,7:2:1) and on cellulose plates Merck using P1, P2 and P4 as solvent systems. The flavonoid compounds were observed under UV light at 366nm before and after visualization by 1% Naturstoffreagenz A in MeOH (NA). The column chromatography (CC) was performed using cellulose Whatman CF 11 Whatman, lipophilic Sephadex (25-100|im). The column elution solvents were: EtOAc-MeOH-H2O (S1,100:6:20, upper layer), EtOAc-MeOH-H2O (S2,100:5:5) and methanol (S3), water (S4), water saturated with ethyl acetate (S5), ethyl acetate saturated with water (S6).
Plant material
The flowers of Trollius europaeus L. were collected from flowering plants growing in the experimental field ofthe Department of Botany, Poznan University of Life Sciences, Poland. The plant was kindly verified by Dr. Wojciech Antkowiak (Department of Botany, Poznan University of Life Sciences). The voucher specimen (Antkowiak, 1033) was deposited at the Department of Pharmacognosy, Poznan University of Medical Sciences.
Extraction and isolation
Five hundred gram of air-dried powdered flowers of Trollius europaeus were extracted with methanol three times at room temperature and, then, exhaustively with the mixture of methanol and water (1:1) on a boiling water bath under reflux. The obtained MeOH and MeOH: H2O extracts, containing the same flavonoids (PC and TLC analysis; P1, P2, P3) were combined, concentrated under reduced pressure, and suspensed in hot water. The resulted precipitate was filtered off. The aqueous fraction (Extract TE) was subjected to CC on the cellulose Whatman CF 11.
The column was initially eluted with S1, followed by S2 and the elution was monitored by PC (P1 and P2), and TLC (P3). 201 fractions contained flavonoids were collected, ca. 200-250ml each. The fractions which had similar constituents were combined. Fraction 1-22 was crystallized from MeOH (S4) to yield 65mg ofyellow needles of compound(6), which was identified as 4'-O-a-rhamnopyranosyl (1^2)-P-xylopyranoside of 7-O-methylapigenin. This compound had also been identified in the leaves of T europaeus [3]. Fraction 23-29 was subjected to Sephadex LH-20 column chromatography (first column with MeOH, next with H2O as eluents) to yield 8mg of compound 7 and 6mg of compound 8 as amorphous powders. These compounds were identified as vitexin (7) and isovitexin (8). Fraction 30-42 was purified using Sephadex LH-20 column chromatography with MeOH (S4) and H2O (S3) as eluents to yield 8mg of yellow crystalline needles of compound 1. Fraction 43-56 was subjected to column chromatography with cellulose, eluted with H2O, saturated with EtOAc (S5) to give 36 fractions. Fraction 1-14 was purified using Sephadex LH-20 column chromatography, eluted with MeOH (S4) to 6mg of 9 and 6mg of 10. Fraction 15-36 was further separated by cellulose CC, eluted with EtOAc saturated with H2O (S6) to yield 39 fractions, which were rechromatographed on a column with Sephadex LH-20, eluted by MeOH (S4); next, it was rechromatographed on a column with Sephadex LH-20, eluted with H2O (S3). As a result, yellow crystalline needles of compound 4 (19mg) were obtained.
Fraction F57-F98 was separated by Sephadex LH-20 column chromatography, eluted with MeOH (S4), and the obtained 11 flavonoid fractions. Fraction 1-7 was subjected to Sephadex LH- 20CC, eluted with H2O (S3), to give 5mg of compound 2. Fraction 8-11 was purified on columns with Sephadex LH-20, first eluted with MeOH (S4), next with H2O (S3) and finally with MeOH (S4), to provide 36mg of yellow crystalline needles of compound 6. Fraction F99-F143 was applied on a Sephadex LH-20 column. Fractionation was carried out by elution with MeOH (S4) to yield 36 fractions. Fraction 4-27 was re- chromatographed on Sephadex LH-20(H2O saturated with EtOAc-S5), and next rechromatographed on Sephadex LH-20 with H2O(S3) as the eluent, yielding 11mg of compound 3 as yellow crystalline needles.
Fraction F144-F201 was separated in the same way as fraction F99-F143. As a result of re-chromatography, 16 mg of compound 5 were obtained as yellow crystalline needles (Figure 1).
Acid hydrolysis
The obtained compounds 1-10 were subjected to hydrolysis with 2% HCl at 100oC for 4h. The hydrolyzates were extracted with EtOAc and the organic fraction was analyzed by TLC (cellulose) and PC P1 and P2, whereas the water fractions were evaporated and analyzed by TLC (cellulose and silica gel 60) with P4 and aniline phthalate/105oC for detection of sugars. The products of the hydrolysis were identified by comparison with the authentic sample: orientin (luteolin 8-C-glucoside) and isoorientin (luteolin6- C-glucoside) for 1-5, vitexin (apigenin 8-C-glucoside) and isovitexin (apigenin 6-C-glucoside) for 7-10, apigenin for 6, andadditionaly,xylose for3, 6, rhamnose for 6,arabinosefor4, 9, glucosefor 5 and galactose for10.
Spectroscopic data
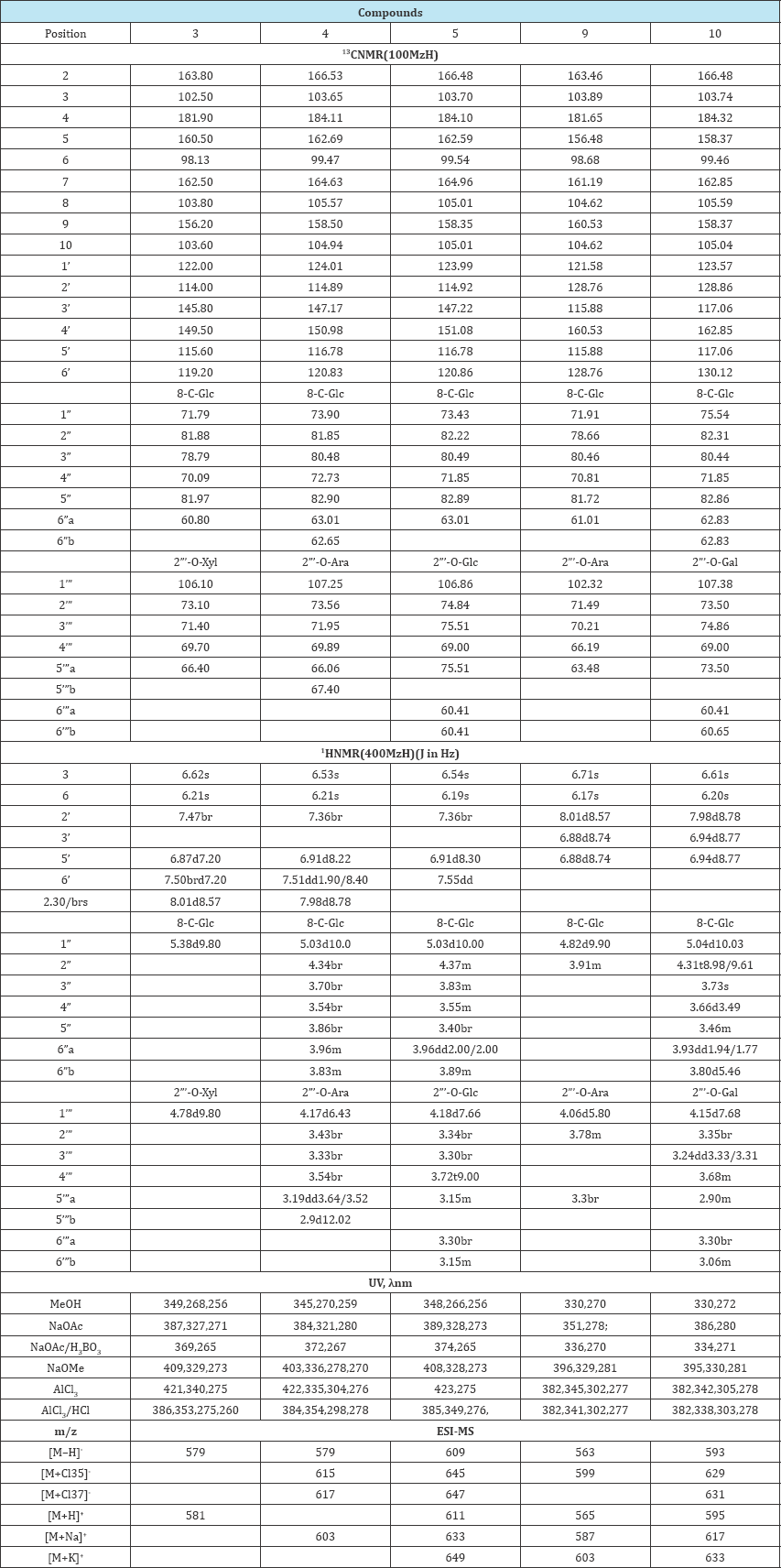
Compounds1, 2, 3, 6, 7, 8were identified by comparison of their spectral data with literature values and confirmed by co-chromatography experiments with authentic standards [3,8-10] . Spectral data of compounds 3, 4, 5, 9, 10 are in (Table 1).
Figure 1: Isolation of flavonoid compounds from Trollius europaeus extract.
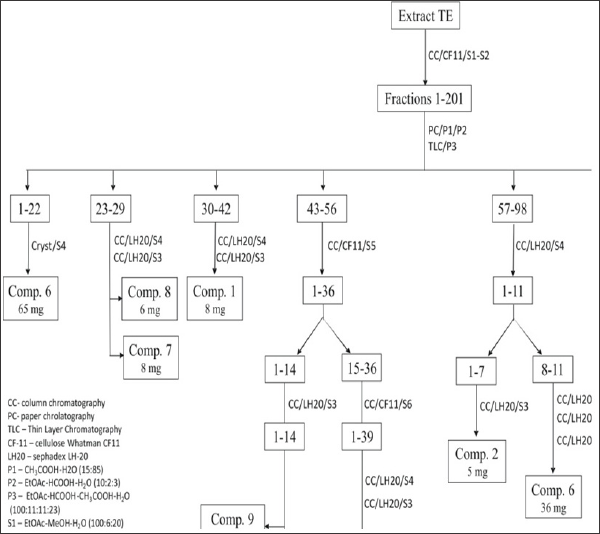
Table 1: Spectroscopic data of compounds 3, 4, 5, 9, 10.
Bioassay
The free radical scavenging capacity was determined in vitro, by using the DPPH (1,1-diphenyl-2-picrylhydrazyl radical) assay [11-13]. The compounds 4, 5, 9, 10 were dissolved in methanol at different concentrations (0.5 - 5.0mg/ml) and mixed with 3.9 ml of 2.2-diphenyl-1-picrylhydrazyl (DPPH) (6,0 x 10-5 mol/L in MeOH). After 30 min of incubation in darkness at room temperature, absorbance (A) was measured at 517nm against a blank.
The free radical activity was calculated by percent inhibition using the following formula:
1% = (Ablank -Asample /Ablank ) x 100, where Ablank was the 'absorbance of the control (containing all reagents except the tested extracts) and Asample was the absorbance of the sample. The resultssample were also expressed using the IC50 parameter, which is defined as the concentration of an antioxidant that causes a 50% DPPH loss of the DPP Hradical scavenging activity. The IC50 parameter was obtained by linear regression. BHA was used as the positive control (0.5-5.00mg/ml).The results have been presented in Table 2.
The data obtained in this study were expressed as the mean of six replicates, plus or minus the confidence interval. The significant difference was considered at the p value <0.05. The statistical analysis was performed using the Microsoft Excel 2007 software.
Preparation of the Extracts
The dried and powdered flowers of Trollius europaeus (5g) were extracted twice with 100ml of methanol-water. (1:1) for 30min using an ultrasonic bath (Elma S 180H, Germany). The extraction was carried out at a temperature of 50oC, 1000W ultrasonic powder, and frequency of 37kHz. The extracts were condensed to dry matter. The dry residue was weighed and dissolved in water thus obtaining 2.5% stock solution.
Determination of total flavonoid content (TFC)
The total content of flavonoids (TFC) was determined using the boric acid colorimetric method described in Pharmacopoeia Polonica [14]. 5ml of the stock solution were evaporated to dryness and the remains were dissolved in 10mL of a mixture of methanol and glacial acetic acid (1:10), 10ml of the solution of boric acid (25g/L) and oxalic acid (20g/L) in anhydrous acetic acid were added, then anhydrous acetic acid was added to obtain 25mL. The reference was prepared in the same way, but the mixture of boric and oxalic acids were replaced with anhydrous formic acid. The absorbance was measured at λ =401nm, after 30min. of incubation. The total flavonoid content was calculated with the following formula: X=Ax 0.8/m, calculated as vitexin, adopting absorbance typical of vitexin equal to 628. The A in the formula is the absorbance of the tested samples, m- the mass of the substance to be examined, in grams.
Tyrosinase inhibitory activity assay
Mushroom tyrosinase, L-dopa used for the bioassay was purchased from Sigma-Aldrich, Chemical Co [14] and hydroquinone from POCH S.A. Poland. The tyrosinase inhibitory activity was measured by spectrophotometry, as described by Bendaikha S et al. [4]. The studies were conducted at a constant temperature of 20oC ±1. The study was conducted for a flavonoid standardized methanol-water extract from the flowers. Five to twenty five ml of the stock solution were condensed to dryness, and then dissolved in 1ml of H2O.
The hundred μL of these concentrations were placed in flasks and then 250μL of 192U/ml of mushroom tyrosinase in phosphate buffer solution (pH 6.8) were added. After pre-incubation at 25 oC for 20 minutes, 200mL of L-dopa (2mM in H2O) were added. The assay mixture was incubated at 25 oC for 10 minutes and the absorbance at 475nm was measured with a spectrophotometer. Hydroquinone, a known tyrosinase inhibitor, was used as a positive control agent. The tyrosinase inhibitory activity was calculated according to the following equation: % inhibition = [(A-B)/A]x 100, where A and B was the absorbance of the enzyme without and with the test material. All the tests were conducted in triplicate. IC50 values were calculated by interpolation of the concentration % inhibition curve by the MS Excel program.
Statistical analyses
The data obtained in this study were expressed as the mean of six replicate determinations plus or minus the confidence interval. The Kruskal-Waallis test was used to assess the significance of the effects of the activity, at p≤0.05. Individual differences between the treatments were identified using the Dunn's test. The correlation of the samples that provided 50% inhibition (IC50) was obtained by interpolation from a linear regression analysis. The calculation was performed using the STATISTICA 10.0 software (Poland).
Results and Discussion
Structural elucidation of flavonoids
The result of this investigation was isolation of flavonoids from the hydromethanolic extract of T. europaeus flowers, with the use of column chromatography. The chromatographic separation was monitored on TLC or PC plates under UV 366 nm. The isolated compounds showed brown fluorescence (with Naturstoffreagenz A) changing to orange (1 -5) or yellow (6-10). The structures of the compounds were established on the basis of the results of acid hydrolysis, ultraviolet spectroscopy (UV), mass spectrometry (MS), and nuclear magnetic resonance (1H and 13C NMR, 1H-1H COSY, HMBC, HSQC NMR) analyses.
Compounds 1-5 and 7-10 were hydrolysed in a way typical of C-glycosides. Under the conditions of acid hydrolysis, compounds 3-5 and 9-10 first underwent separation of the sugar molecule attached to the O-glycosidic bond (arabinose from 9 and 4, xylose from 3, glucose from 5 and galactose from 10) and, then,interconvertion (i.e. Wessely-Moser rearrangement) to a mixture of 6- and 8-isomers (orientin and isoorientin for 1-5, vitexin and isovitexin for 7-10). The Wessely-Moser rearrangement in the same conditions was also observed for compounds 1, 2, 7,8 which possessed the sugar molecule attached by a C-glycosidic bond [7,15].
For compound 6, the acid hydrolysis was characteristic of di- O-glycosides of flavonoids. The conditions of the hydrolysis first led to the separation of the outer sugar molecule (product-mono- glucoside of flavonoid) and, next, of another one until an aglycone were obtained. The results of the chromatographic analysis of the hydrolysis products of compound 6 indicated the presence of two simple sugars corresponding to xylose and rhamnose and an aglycone with standard 7-methylapigenin.
The analysis of the UV spectra see data in Table 1 indicated that compounds 1-5 were flavones with free OH group sat C-3', 4'; 5 and 7, while compounds 7-10 were flavones with free OH groups at C-4', 5 and 7. Compound 6 was a flavone with one free OH group at C-5 [7]. In the ESI- MS spectra of compounds 1- 10 protonated and deprotonated molecules of the compounds and their adducts with sodium, potassium and chlorine were observed in the positive and negativeion modes. Based on the results of the ESI-MS analysis, the mass of compounds 1/2=m/z 448, 3=m/z 580, 4 = m/z 580, 5 = m/z 610, 6 = m/z 562, 7/8 = m/z 432, 9 = m/z 564, 10 = m/z 594 was determined. The results indicated that the mass of compounds 1 and 2 corresponded to luteolin substituted with hexose, that of compounds 3 and 4 corresponded to orientin substituted with pentose (C26H28O15), 5 was compatible with orientin substituted with hexose (C27H30O16). Compounds 7 and 8 indicated to apigenin substituted with hexose, while compound 9 was equivalent to vitexin with bound pentose (C26H28O14) and compound 10- to vitexin substituted with hexose (C27H20O21). The resonance signals from all the carbons and protons could be assigned by analyzing the 1H-1H COSY and 1H-13C HMQC, 1H-13C HMBC spectra. In the 1H NMR spectrum ofcompounds3, 4, 5 there were singlets from H-3 and H-6 typical of orientin derivatives that appeared in the range of δ 6.19ppm to δ 6.62ppm, a doublet of doublets with the coupling constant about 2.0/8.0Hz or broad signals from H-6'. The H-5' signals were seen in the form of doublets in the range of δ 6. 87ppm to δ 6.91ppm with the coupling constant about 8.0Hz. Signals from H-2' appeared as broad signals but there was no signal from H-3' [9, 11]. No signal in the region ofH-8 signals (δ6.39 to 6.56ppm) indicated the presence of a substituent at C-8. In the 13CNMR spectrum, signals of C-8were shifted down field by about 10ppm, compared with luteolin, indicating that sugar linked at C-8 by a C-glycosidic bond. In the region of anomeric sugar protons there were doublets of the sugar molecule in the range of δ5.03ppm to δ 5.38ppm (d,H-1”, J=10.0Hz) attached directly to the aglycone, and from the outside of the sugar molecule at δ4.78ppm (d, H-1”', J=9.8), δ4,17ppm (d, H-1”', J=6.43Hz) and δ4.18ppm (d, H-1”', J=7.66Hz), respectively. The constant coupling signals from the anomeric protons of the sugars showed that they occurred in the β configuration. The correlation occurring between the protons and carbons was determined on the basis of 1H-13C HSQC). The anomeric carbon of glucose, which constituted the internal sugar molecule, disclosed in the range of δ71.79 to 73.90ppm, whereas the one of the sugar attached to glucose in the range δ106.10 to 107.25ppm. It was observed that the C-2” resonance signal of glucose was shifted downfield by about 10ppm, while the signals of the adjacent carbons were shifted up field, which suggested substitution of C-2” glucose with a sugar molecule (Table 1). [15,16] The values of the signals and their shifts for the investigated compounds were comparable to the results of the identification analyses described for orientin 2”-O-β-arabinopyranoside isolated from T ledebouri [9], Deschampsia antarctica[17], and for orientin 2”-O-β-glucopyranoside isolated from Cannabissativa, Seteria italic [8].
In the HMBC spectrum of compound 4 correlations observed among H-2” and C-1”' confirmed the connection 1→2 between the sugar molecules. The anomeric proton of glucose was correlated with C-7 and C-9 of the aglycone. H-3 showed cross peaks with C-10, C-1', C-4' and H-8 showed HMBC correlation with C-6, C-9 and C-10, as well as H-6 to C-5, C-8 and C-10, confirming the positions of these hydrogens. In the HMBC spectrum of 5, the long-range correlations between H-1” of glucose and C-7, C-9, C-10 indicated that glucose was attached to the C-8 position of the aglycone. Correlations between C-2”and H-1”' suggested 1→2 interglycosidic linkage. The long-range correlations from H-6 to C-5, C-8, C-10, as well as between H-3 and C-8, C-10, C-1', C-4', confirmed the positions of these hydrogens. The hydrogens of ring B correlated in the following manner: between H-2' and C-2, C-4', also between H-5' and C-1', C-3', C-4', and from H -6' to C -2', C-3', C-4'. The 1H NMR spectrum of compound 9 and 10, in the region of the aglycone, revealed signals typical of a pigenin: singlets from H-6 at δ6.2ppm for compound 9 and at δ6.17ppm for compound 10, from H-3 at δ6.61ppm for compound 9 and at δ6.71ppm for compound 10, and doublets from H-2' and H-6' at 57.98ppm and δ8.1ppm, respectively, whereas for H-3' and H-5' at δ6.94ppm and δ 6.88ppm, respectively. The doublets were characterized by coupling constants about 9.0Hz.
Additionally, no signal of proton H-8, which should have appeared at δ6.39 to δ6.56ppm, and the signal shift at C-8 in the 13C NMR spectrum compared to the corresponding signal of apigenin, may suggest that compound 9 and 10 had a substituent at C-8. In the region of the anomeric proton signals doublets at δ 4.15ppm for compound 9 and δ4.82ppm for 10 with the coupling constants about 10.0, indicated β configuration. In the 13C NMR spectrum, in the range of the signals of the anomeric carbon sugar molecules, diagnostic signals of glucose, and of arabinose for compound 9 and of galactose for compound 10, were observed. The signal from C-1” glucose appeared at δ71.91ppm for compound 9, and at δ 102.32ppm for arabinose. For compound 10 the signal at δ75, 54ppm was assigned to glucose, whereas the signal at δ107, 38ppm was assigned to galactose. In relation to vitexin, the signal of the C-2" carbon for both of the compounds was shifted downfield by about 10ppm, which indicated the presence of outer sugar on the second carbon of glucose. The analysis of the spectrum of compounds 9 and 10, especially the sugar part, just like the one of compounds 4 and 5, was hampered by the presence of multiple signals in a narrow range of the spectrum (signals of δ5.04ppm to δ2.90ppm in the 1H NMR and carbons of δ 107.38ppm to δ60.41ppm in the 13C NMR). Two further doublets of sugar protons were assigned to anomeric protons of the outer sugar molecule [9]. In the HMBC spectrum of 10, the long-range correlations between H-1" of glucose and C-7, C-8, C-9, C-10 indicated that glucose was attached to the C-8 position of the aglycone. The long-range correlations between H-6 and C-5, C-7, C-8, C-10 confirmed the positions of these hydrogens. The hydrogens of ring B correlated in the following manner: between H-2' and C-2, C-4', as well as between H-5' and C-2, C-1', C-4'; there were also correlations between H-6' and C-2 and C-4', and between H-3' and C-1', C-4'. The link between the sugar units was established by a HMBC cross signal between H-1"' of galactose and H-2" of glucose. The results of the identification analyses indicated that the isolated compound 9 was vitexin 2”-O-β-arabinopyranoside and has been isolated in the plant world for the first time. Compound 10 was identified as vitexin 2"-O-β-galactopyranoside. This compound had also been isolated and identified in T ledebouri and T chinensis. [10, 16-20]
The analysis of the studies conducted (acid hydrolysis, ultraviolet spectral analysis, mass spectrometry and the results of the spectral analysis of the nuclear magnetic resonance) indicated that compound 3 was orientin 2"-O-p-xylopyranoside (adonivernith). The resonance signals were consistent with the ones determined for orientin 2"-O-β-xylopyranoside (adonivernith)[9] . Adonivernith was isolated from different organs of Trollius europaeus in studies conducted simultaneously by Gallet et al. [21]. Based on the results, compound 4 was determined to be orientin 2"-O-β- arabinopyranoside, compound5 was identified as orientin 2"-O-β-glucopyranoside.
These spectral and hydrolysis data suggested that compound 9 was vitexin 2"-O-β-arabinopyranoside, whereas 10 vitexin 2"-O-β-galactopyranoside. The vitexin 2"-O-β-arabinopyranoside was isolated from the plant world for the first time. The C-glycosylflavones, i.e. orientin (1), isoorientin (2), vitexin (7) and isovitexin (8), were identified on the basis of their structural assignments (1H and 13C NMR and ESI-MS, UV-spectrophotometric analysis). Their spectroscopic data were in good agreement with those reported in the literature [6, 10].
4'-O-α-rhamnopyranosyl (1→2)-β-xylopyranoside of 7- O-methylapigenin (6), identified in the flowers, had been previosuly isolated from and identified in the extract from the leaves of T. europaeus. This spectroscopic data (1H and 13C NMR and ESI-MS, UV-spectrophotometric analysis) were in good agreement with those reported in my first study [3].
Investigation of the antioxidant activity
The antioxidant activity of the compounds isolated from the Trollius flowers was investigated using the DPPH assay and the results were analysed statistically by means of the Kruskal- Wallis test, post hoc Dunn's test [11-13]. The results indicated that compounds 4 and 5 showed significant DPPH free radical scavenging activity. The activity of compound 4 was comparable to that of BHA. The IC50 values of compounds 4 and 5 were 1.67mg/mL, 3.41mg/mL, respectively, whereas the IC50 for 9 and 10 amounted to 142.9mg/mL and 576.5mg/mL, respectively. The IC50 of BHA, used as a standard, was 1.67mg/mL.
The statistical analysis showed that for all the investigated compounds there was a significant difference in activity between the concentration of 0.5mg/mL, 1mg/mL and 5mg/mL; the same relation was observed for the concentration of 0.5mg/mL and 4mg/mL for compounds 4, 5, 9, and BHA. A comparison of the investigated compounds revealed that the derivatives of both orientin and vitexin substituted with a molecule of arabinose demonstrated significantly stronger activity than the derivatives of orientin and vitexin substituted with hexose (glucose, galactose, respectively) (Table 2).
Table 2: DPPH radical scavenging activity (%) of compounds 4, 5, 9,

The data indicated that the presence of hydroxyl groups at the aglycone could enhance the antioxidant activity of the flavonoids, as orientin showed greater scavenging activity than vitexin. The results of previous studies suggested stronger antioxidant activity of orientin 2"-O-xyloside from Setariaviridis (Gramineae) than that of vitexin 2"-O-glucoside, while vitexin 2"-O-xyloside was inactive [22]. Orientin 2"-O- galactoside (OGA), separated from T. chinensis flowers, showed greatest scavenging activity against DPPH (IC50=23.9μg/ml) and was followed by orientin and vitexin which demonstrated moderate scavenging activity against DPPH (112.4μg/ml and 217.9μg/ml, respectively). It was observed that the tested compounds showed weak or no scavenging activity against ABTS + By comparison with orientin, OGA showed particularly strong scavenging activity in this case, which might be ascribed to its improved polarity and solubility by an additional galactoside moiety [23, 24].
Table 3: nhibitory effects on mushroom tyrosinase of methanol-water extracts from flowers of T. europaeus(TK) and hydroquinone (HQ).
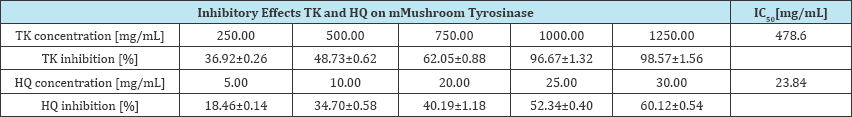
Inhibitory effect of Trollius flowers extract (TK) on mushroom tyrosinase
Tyrosinase is a glycosylated multi-copper mono oxygenase enzyme. It is responsible for the pigmentation of eyes, hair, and skin. It contributes to undesired browning of fruits and vegetables. The changes in pigmentation in mammal organisms (hypo-, hyperpigmentation), as well as fruit and vegetable browning, may be connected with disorders of tyrosinase activity. This has prompted scientists to search for new, safe inhibitors of the enzyme for use in foods and cosmetics (Table 3).
The antityrosinase activity was determined for a methanol- water extract from the flowers of T europaeus (TK) standardised for the content of flavonoids calculated as vitexin. The total content of flavonoids in the investigated extract was 0.4±0.03%. The determination of tyrosinase inhibition was performed with the use of the mushroom tyrosinase assay for different concentrations of the TK extract corresponding to 1250.00 to 250mg/ml of the raw material. The IC50 of the TK extract was 478.6mg/ml, whereas for hydroquinone HQ used as the positive control it was 23.84mg/ml. The extract from the flowers of T europaeus containing 0.4% of flavonoids also seems a potentially good factor of antityrosinase. This ability may be related to the structural similarity of polyphenols to L-DOPA and tyrosine, which are natural substrates for tyrosinase [25].
Acknowledgment
I thank Wiesiawa Bylka (Department of Pharmacognosy, Poznan University of Medical Sciences), I am grateful and indebted to her for her expert, sincere and valuable guidance. I sincerely thank Wojciech Antkowiak (PhD, Department of Botany, Poznan University of Life Sciences) for providing the plant material for the research.
References
- Gruenwald J, Brendler T, Jaenicke C (2004) PDR for herbal medicines. Thomson PDR, USA, pp. 387-388.
- Maciejewska R, Antkowiak W, Andrzej MJ, Ewa WB, W Bylka (2007) Chemical composition and morphology of basal leaves of Trollius europaeus L. and T. altissimus Crantz (Ranunculaceae). Pol J Environ Stud 16(4): 595-605.
- Witkowska Banaszczak E, Bylka W (2015) New flavonoid compound of Trollius europaeus. Acta Physiologiae Plantarum 37: 99.
- Bendaikha S, Gadaut M, Harakat D, Magid A (2014) Acylated flavonol glycosides from the flower of Elaeagnus angustifolia L. Phytochemistry 103:129-136.
- G^sowska Bajger B, Wojtasek H (2014) Errors in the study of enzymatic reactions resulting from unforseen oxidation-reduction reactions. Chemik 68(4): 341-346.
- Kim D, Park J, Kim J, Han C, Yoon J, et al. (2006) Flavonoids as mushroom tyrosinase inhibitors: a fluorescence quenching study. J Agric Food Chem 54(3): 935-941.
- Mabry TJ, Markham KR, Thomas MB (1970) The systematic identification of flavonoids. Springer, USA, pp. 1-164.
- Harborne JB (1995) The flavonoids: Advances in research since 1986. J Chem Educ, USA, pp. 1-718.
- Zou JH, Yang JS, Dong YS, Zhou L, Lin G (2005) Flavone C-glycosides from flowers of Trollius ledebouri. Phytochemistry. 66(10): 1121-1125.
- Andersen QM, Markham KR (2006) Flavonoids: chemistry, biochemistry and applications. Taylor & Francis Group, USA, pp. 1-1256.
- Brand Williams W, Cuvelier ME, Berset C (1995) Use of a free radical method to evaluate antioxidant activity. LWT - Food Science and Technology 28(1): 25-30.
- Molyneux P (2004) The use of the stable free radical diphenylpicryl- hydrazyl (DPPH) for estimating antioxidant activity. Songklanakarin J Sci Technol 26(2): 211-219.
- Assimopoulou AN, Sinakos Z, Papageorgiou VP, et al. (2005) Radical scavenging activity of Crocus sativus L. extract and its bioactive constituents. Phytother Res 19(11): 997-1000.
- (2014) Pharmacopoeia Polonica. (10th edn), pp. 1388-1389.
- Dou M, He XH, Sun Y , Peng F , Liu JY, et al. (2015) Controlled acid hydrolysis and kinetics of flavone C-glycosides from trollflowers. Chinese Chemical Letters 26(2): 255-258.
- Zhou X , Peng J, Fan G, Wu Y (2005) Isolation and purification of flavonoid glycosides from Trollius ledebourii using high-speed counter-current chromatography by stepwise increasing the flow-rate of the mobile phase. J Chromatogr A 1092(2): 216-221.
- Webby RF, Markham KR (1994) Isoswertjaponin 2”-O-p- arabinopyranoside and other flavone-C-glycosides from the antractic grass Deschampsia antarctica. Phytochemistry; 36(5): 1323-1326.
- Wu XA, Zhao YM, Yu NJ (2007) Flavone C-glycosides from Trollius ledebourii reichb. J Asian Nat Prod Res 8(6): 541-544.
- Wu LZ, Wu HF, Xu XD, Yang JS (2011) The new flavone C-glycosides from Trollius ledebourii . Cem Pharm Bull 59(11): 1393-1395.
- Cai SQ Wang R, Yang X, Shang M, Ma C, et al. (2006) Antiviral flavonoid- type C-glycosides from the flowers of Trollius chinensis. Chem and Biodivers 3(3): 343-348.
- Gallet C, Ibanez S, Zinger L, Taravel FR, Trierweiler M, et al. (2007) Plant chemical defense included by a seed-eating pollinator mutualist. J Chem Ecol 33(11): 2078-2089.
- Kwon YS, Kim EY, Kim WJ, Kim WK, Kim CM, et al. (2002) Antioxidant constituents from Setariaviridis. Arch Pharm Res 25(3): 300-305.
- Sun Y, Yuan H, Hao L, Min C, Cai J, et al. (2013) Enrichment and antioxidant properties of flavone C-glycosides from trollflowers using macroporous resin. Food Chem 141(1): 533-541.
- Yuan M, An YN, Wang RF, Ding Y, Sun ZX (2014) Distribution of two bioactive compouds in flowers of Trollius chinensis. J Chromatogr Sci 52(5): 466-469.
- Karioti A, Protopappa A, Megoulas N, Skaltsa H, et al. (2007) Identification of tyrosinase inhibitors from Marrubium velutinum and Marrubium cylleneum. Bioorg Med Chem 15(7): 2708-2714.
© 2018 Ewa Witkowska Banaszczak Orcid. This is an open access article distributed under the terms of the Creative Commons Attribution License , which permits unrestricted use, distribution, and build upon your work non-commercially.
 a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in
a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in 







.jpg)






























 Editorial Board Registrations
Editorial Board Registrations Submit your Article
Submit your Article Refer a Friend
Refer a Friend Advertise With Us
Advertise With Us
.jpg)






.jpg)














.bmp)
.jpg)
.png)
.jpg)










.jpg)






.png)

.png)



.png)






