- Submissions

Full Text
Modern Applications in Pharmacy & Pharmacology
Thermal Methods of Analysis
Nirav Soni*
Department of Quality Assurance, India
*Corresponding author: Nirav Soni, Department of Quality Assurance, India
Submission: October 19, 2017; Published: December 22, 2017

ISSN 2637-7756Volume1 Issue2
Content
a. Fundamental Definition.
b. Theory.
c. Instrumentation and Applications of Thermo Gravimetric Analysis (TGA).
d. Differential Thermal Analysis (DTA).
e. Differential Scanning Calorimetry (DSC).
f. Thermo Mechanical Analysis (TMA) (Figure 1).
Figure 1:
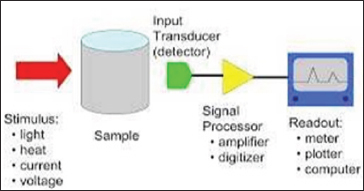
Fundamental Definition
Thermogravimetry (TG)
The mass of a sample is measured as a function of the temperature, which increases linearly e.g. from RT to 1200 °C.
Calorimetry
It is the process in the study of heat transfer during physical and chemical processes
a. A device for measuring the heat transferred.
b. It was found Lavoisier and Laplace (1782 -1784).
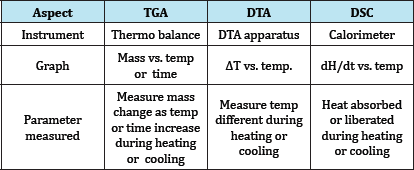
TGA and DSC
I. Thermo Gravimetric Analysis (TGA)
a. Mass change of a substance measured as function of temperature the substance is subjected to a controlled temperature program.
b. Mass is lost if the substance contains a volatile fraction.
II. Differential Scanning Calorimetry (DSC)
a. Provides information about thermal changes that do not involve a change in sample mass.
b. More commonly used technique than TGA.
c. Two basic types of DSC instruments: heat-flux and power compensation.
Thermal Methods
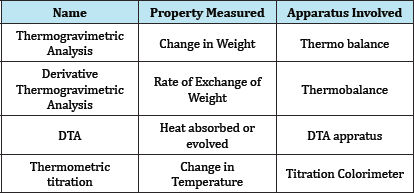
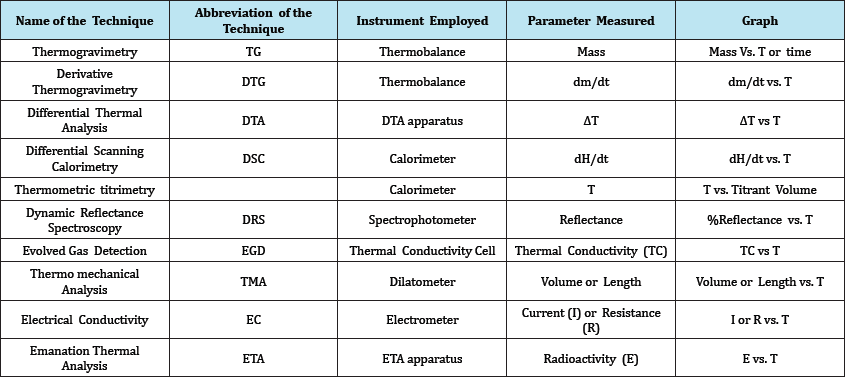
Thermal analysis incorporates those techniques in which some physical parameter of the system is determined and recorded as a function of temperature (ΔT). Measurements based on dynamic relationships between temperature and Mass, Volume, Heat of reaction. Thermal methods are as following
a. Thermo Gravimetry Analysis (TGA).
b. Derivative Thermogravimetric Analysis (DTG).
c. Differential Thermal Analysis (DTA).
d. Differential Scanning Calorimetry (DSC).
e. Thermometric Titration (Table 1).
Table 1: Classification of analytical method.
Thermogravimetric Analysis
Definition
It is a technique where by the weight of substance, in an environment heated or cooled at a controlled rate, is recorded as a function of time or temperature. A large number of chemical substances incariably decompose upon heating and this idea of heating a sample to observe weight changes is the underlying principle of TGA. Three Types of TG:
a. Isothermal or static TG (Constant T).
b. Quasistatic TG (Constant weight).
c. Dynamic TG (T changing in linear rate).
Principle
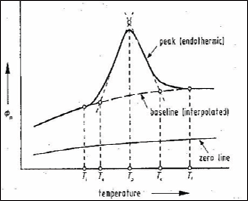
The principle of the technique can be illustrated by the weight loss curve of compound (Figure 2 & 3).
Figure 2: Weight loss curve of compound.
AA': compound is stable.
A'B': weight loss.
Ti: Procedural decomposition temperature (Lowest T at which the cumulative mass change reaches a magnitude that the thermo balance can detect).
Tf: Final temperature.
Ti-Tf: Reaction interval.
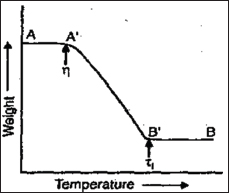
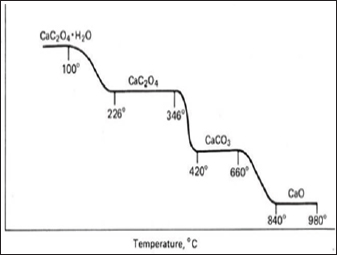
Figure 3: Temperature vs ΔW (weight loss) calcium oxalate.
Basic principles of thermal analysis
Modern instrumentation used for thermal analysis usually consists of four parts:
a. Sample/sample holder.
b. Sensors to detect/measure a property of the sample and the temperature.
c. An enclosure within which the experimental parameters may be controlled.
d. A computer to control data collection and processing.
Instrumentation
Two Techniques of Analysis are generally adopted.
a. Dynamic TGA: Continuous increased in Temperature Linear with Time.
b. Isothermal TGA: Maintained Constant Temperature for a given time during which its weight is recorded.
Differential Thermal Analysis (DTA)
Principle
Differential thermal analysis (DTA) involves the technique of recording the difference in temperature between a substance and a reference material against either time or temperature. Thus, a differential thermogram consists of a record of the differences in sample and reference temperature (differential temperature, AT) plotted as a function of time t, sample temperature (Ts), reference temperature (Tr) or furnace temperature (Tf). Substance and reference material are subjected to controlled temperature
a. Emissions of heat (exothermic).
b. Absorptions of heat (endothermic) (Figure 4).
Figure 4: Principle.
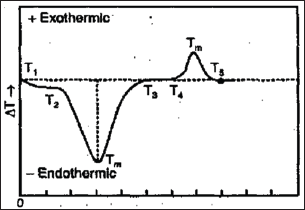
Sharp endothermic peaks give ideas of changes in crystallinity or fusion processes whereas broad endosperms signify dehydration reactions. Physical changes give rise to endothermic curves whereas chemical reactions (particularly those of an oxidative nature) give rise to exothermic peaks. DTA allows the detection of every physical or chemical change whether or not it is accompanied by a change in weight.
Factors affecting the DTA curve
The DTA curve is affected by a larger number of factors than the TG curve. Majority of these factors are common to TG and hence will not be discussed but only their special influences on DTA curves will be considered. The various factors affecting die DTA curve are as follows:
a. Environmental factors.
b. Instrumental factors.
c. Sample factors.
Environmental factors: The DTA technique is more sensitive to the gaseous environment around the sample. The reaction of gaseous atmosphere with the sample may also produce extra peaks in the curve. For example
a. Oxygen in air which causes an oxidation reaction may give rise to an exothermic peak.
b. If we record the DTA of lignite in the dynamic nitrogen atmosphere, it pyrolyses and distils off volatile matter. However, in the dynamic oxygen atmosphere, the lignite oxidizes, resulting exothermic instead of endothermic peaks.
Instrumental factors
a. Sample holder: The geometry and the material used in the fabrication of the sample holder affect the resolution, shape and size of the DTA peaks. For better resolution, the size of holders and the amount of sample should be as small as possible.
b. Differential temperature sensing devices: The positions of the temperature measuring the differential thermocouples affect the intensity of the peaks, shapes of the peaks and the base line. However, one gets best results when the differential temperature is plotted against the sample temperature.
c. Temperature-programmer controller: One should be quite careful while selecting temperature- programmer controller because a constant heating rate is required in DTA.
d. Thermal regime: The heating rate has a great influence on the DTA curves. Higher the heating rates, higher the peak temperatures and sharper the peaks with greater intensity. Heating rates of 10 to 20 °C per minute are employed.
e. Recorder: DTA curve is greatly influenced by the type, chart-speed and pen-response of a recorder.
Sample characteristics
a. Physical: The degree of crystallinity of the sample also affects the DTA curves. The weight of the sample also influences the peak intensity and temperatures. Both these increase with increasing weight. Particle size.
b. Chemical: The chemical reactivity of the sample, the sample holder, thermocouple material, the ambient gaseous environment and added diluents greatly alter the DTA peaks (Figure 5).
Figure 5: Instrumentation.
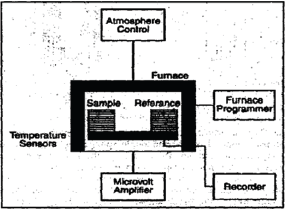
Sample Holders
Material
Both metallic as well as non-metallic materials are employed for the fabrication of sample holders. Metallic materials generally include nickel, stainless steel (up to 1000 °C), platinum and its alloys. Metallic holders give rise to sharp exotherms and flat endotherms. Non-metallic material generally includes glass, vitreous silica or sintered alumina. Non-metallic holders yield relatively sharp endotherms and flat exotherms (Figure 6).
Figure 6: Geometry.
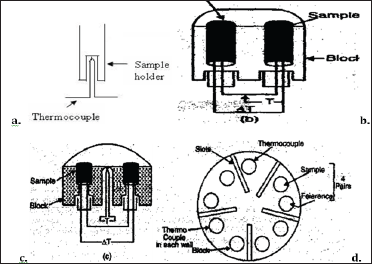
Furnace
a. Always prefer a tubular furnace. This is constructed with an appropriate material (wire or ribbon) wound on a refractory tube.
b. Another important characteristic of furnace is its dimension which depends mainly upon the length of the uniform temperature zone desired.
c. Calculate dimension of the furnace by taking into account the size of me sample holder, length of uniform temperature zone, heating rates, and cost.
Temperature Control
To control temperature, the three basic elements are required.
a. Sensor
b. control element
c. heater
The control element governs rate of heat-input required to match heat loss from the system.
Recorder
The signals obtained from the sensors can be recorded in which the signal trace is produced on paper or film by ink, heating style, electric writing or optical beam. The mode of analog recording is two types:
a. Deflection type: In this the recording pen is moved directly by the input signal.
b. Null type: In this the input signal is compared with reference or std signal and difference is amplified and used to adjust the reference signal until it matches the input signal.
Thermocouples
In DTA temperature sensor are thermocouples. Selecting the thermocouple as the temp sensor following points are consider
a. Temperature interval.
b. Thermoelectric coefficient.
c. Chemical compatibility with sample.
d. Chemical gaseous environment.
e. Availability and cost.
It is made of chromel P and alumel wires are used to measure and control the temp upto 1100 oC in air. Above 1100 oC should use thermocouple made from pure platinum and platinum-rhodium alloy wires.
Cooling Device
The cooling system is considered separate from the temperature programmer because in most instruments cooling is completely independent from heating. A simple automatic cooling system.
Application
Physical chemistry
Heat of reaction: The derivation of expression relating area under a DTA peak to the heat of reaction has been worked out by Borchardt and Daniels. They concluded that the peak area 'A' in DTA is always a linear function of heat of reaction ΔH=KA/NO.
Specific heat: DTA has been employed by David to determine specific heat of substances like naphthalene. The samples do not undergo any thermal effect other than the normal change in specific heat. CP was determined by using the following formula: Cp=KK'(b-a)/dmsdT.
Thermal diffusivity: DTA has been used to determine the thermal diffusivities by measuring the temperature difference AT between the centre and surface of the sample, heated at a uniform rate. The thermal diffusivity. D was determined by using me following relation D=βr2/6 ΔTs
Analytical chemistry
Identification of substances: We know that the DTA curve for two substances is not identical. Therefore, these serve as finger prints for various substances.
Identification of products: When a substance reacts with another substance, the products are identified by their specific DTA curves.
Melting points: It can be easily identified by DTA. So, that method is used directly to check the purity of the compound.
Quantitative analysis: The area of the peak is proportional to the total heat of the reaction. Therefore, the quantitative analysis is possible with the help of the standard curve of peak area vs. weight.
Quality control: DTA technique has been widely used for the quality control of a large number of substances like cement, glass, soil, catalysts, textiles, explosives, resins, etc. The characterization of limestone used in production of Portland cement has been done by DTA. The amount of magnesium carbonate in cements can be controlled by a quantitative analysis of the DTA curve.
Inorganic chemistry: DTA technique has been used to study the thermal stability of a large number of inorganic compound and complexes. DTA technique is preferred because it helps in distinguishing between reversible phase changes and irreversible decompositions. DTA techniques have been used to study oxalates, metal amine complexes, carbonates and oxides.
Organic chemistry: DTA investigations have been carried to help identification, purity determination and quantitative analysis including the evaluation of kinetic parameters of polymers, explosives, pharmaceuticals, oils, fats and other organic chemicals.
DSC
DSC is a thermo analytical technique in which the difference in the amount of heat required to increase the temperature of a sample and reference are measured. Both the sample and reference are maintained at nearly the same temperature throughout the experiment. Differential scanning calorimeter (DSC) is a technique for measuring the energy necessary to establish a nearly zero temperature difference between a substance and an inert reference material
Based on mechanism of operation DSC can be classified into two types
a. Heat flux DSC
b. Power Compensated DSC
Instrumentation Consists of following components
a. Thermo-balance
b. Sample Holder
c. Sensors
d. Furance
Figure 7: Heat flux DSC and power compensated DSC.
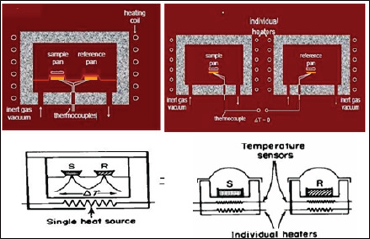
e. Temperature Controller (Figure 7, Table 2).
Table 2: Heat flux DSC and power compensated DSC.
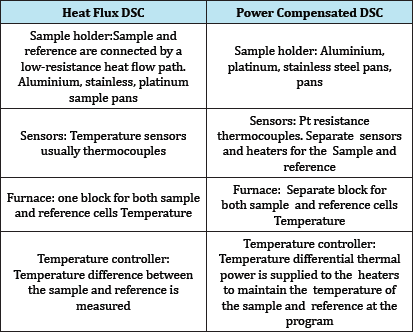
Thermo Balance
This is the most important part of the instrument. Important features:-
a. Accuracy
b. Sensitivity
c. Reproducibility
Generally balance should have following characteristics
a. It could cover wide range of temperature.
b. Should have high degree of mechanical rigidity and electronic stability.
c. Temperature recording should be within ±1 °C.
d. Should have an adequate range of automatic wt. adjustment.
e. Should have high degree of mechanical and electronic stability.
f. Rapid response to wt. change.
g. Unaffected by vibration.
Recording thermo balance type
a. Deflection Thermo balance: Beam type thermo balance ,Helical type thermo balance
b. Null Thermo balance i.e. sensing element attached.
Deflection balances
Change in the sample mass
Deflection of the beam
Imbalance in the photodiode current is amplified
Amplified photodiode current is monitored
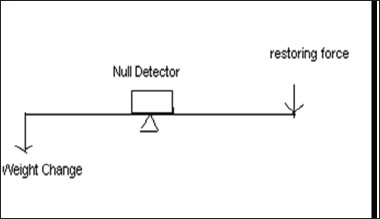
Null point balance: There should be a sensor to detect the deviation of the balance beam from its null position. This deviation is directly proportional to the weight change (Figure 8).
Figure 8: Null point balance.
Sample holder: Aluminium, Platinum , Stainless still pans.
Sensors: Temperature sensors usually Thermocouples. Furance: Made from high Quality metal and vary in shape &
a. Temperature 1000-2000 °C.
b. For higher temperature tungsten or rhenum thermocouples are used.
c. Separate block for both sample and reference Cells.
Temperature controller: Temperature difference between the sample and reference is measured (Table 3).
Table 3: Difference.
Principles
The basic principle underlying this technique is that, when the sample undergoes a physical transformation such as phase transitions, heat or energy will need to maintain both at the same temperature is calculated
For example, as a solid sample melts to a liquid it will require more heat flowing to the sample to increase its temperature at the same rate as the reference. This is due to the endothermic phase transition from solid to liquid. Likewise, as the sample undergoes exothermic processes (such as crystallization) less heat is required to raise the sample temperature. By observing the difference in heat flow between the sample and reference, differential scanning calorimeters are able to measure the amount of heat absorbed or released during such transitions (Table 4 & Figure 9).
Figure 9: Instrumentation.

Table 4: Difference between DSC & DTA.

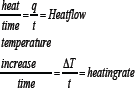
Heat Capacity
When both samples are subjected to heat, the computer will plot the difference in heat output of the two heaters against temperature. Means we're plotting the heat absorbed by the sample against temperature.
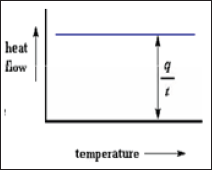
Where t=time, ΔT= increase in Temp, q=heat (Figure 10).
Figure 10: Heat capacity.
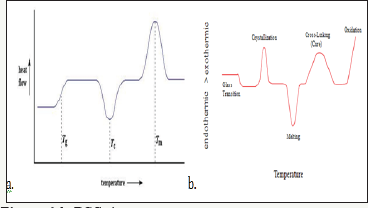
Three Phase of DSC Curve
The glass transition temperature: An endothermic transition. Transition from solid to less viscous form. Positive peak (Figure 11).
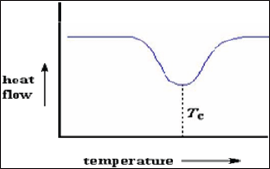
Crystallization: An exothermic transition. Sharp negative peak. Disorder to order transition. For measuring latent heat observed in glassy solid e.g. Polymer (Figure 12).
Figure 11: Glass transition temperature.

Figure 12: Crystallization.
Melting: An endothermic transition. Positive peak. Order to disordered transition (Figure 13 & 14).
Figure 13: Melting.
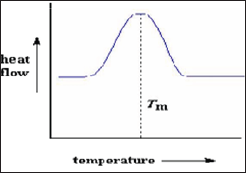
Figure 14: DSC thermogram.
What DSC Can Measure?
a. Glass transitions
b. Melting and boiling temp.
c. Crystallization temp and time.
d. Percentage crystallinity.
e. Heat of fusion and reaction.
f. Specific heat capacity.
g. Oxidative/thermal stability.
h. Reaction kinetic.
i. Purity.
Application
For measuring no. of characteristic of sample by observe fusion and crystallization events as well as glass transition temperatures (Tg). In quantitative analysis finger printing of clays, polymers, minerals. For sample purity melting point. DSC can also be used to study oxidation, as well as other chemical reaction. Using differential scanning calorimetry to study the oxidative stability of samples. In the pharmaceutical industry it is necessary to have well- characterized drug compounds in order to define processing parameters. DSC may also be used in the study of liquid crystals. As matter transitions between solid and liquid it often goes through a third state. This anisotropic liquid is known as a liquid crystalline or mesomorphous state. Using DSC, it is possible to observe the small energy changes that occur as matter transitions from a solid to a liquid crystal and from a liquid crystal to an isotropic liquid. DSC is widely used in polymer industries for studying curing processes, The cross-linking of polymer molecules that occurs in the curing process is exothermic give positive peak. In food science research, DSC is used in conjunction with other thermal analytical techniques to determine water dynamics and determination of oils and fats. For metallic material it is possible to use DSC to find solidus and liquidus temperature of a metal alloy
a. Glass transition temp.
b. Heats of crystallization and fusion.
c. Heats of curing reaction.
d. Heat of decomposition.
e. Heat of vaporization and heats of solution.
DSC curves may also be used to evaluate drug and polymer purities.
DSC calibration
Temperature: Match melting onset temperatures to the known melting points of standards analyzed.
DSC: Should be calibrated as close to desire temperature range as possible.
Heat flow: Use calibration standards of known heat capacity, slow accurate heating rates (0.5-2.0 °c/min), and similar sample and reference pan weights.
Calibrants
a. High purity
b. Accurately known enthalpies
c. Thermally stable
d. Light stable
e. Not hygroscopic
f. Do not react (pan, atmosphere)metals
g. Indium 156.6 °c; 28.45j/g
h. Zinc 419.47 °c, 108.17j/g inorganics
i. Kno3128.7 °c
j. Kclo4299.4 °c organics polystyrene 105 °c
k. Benzoic acid 122.3 °c; 147.3 j/g
Baseline calibration: Evaluation of the thermal resistance of the sample and reference sensors. Measurements over the temperature range of interest (Figure 15).
Figure 15: Baseline calibration.
Sample preparation: Small sample masses and low heating rates increase resolution, but at the expense. Accurately-weighed samples (~3-20mg, usually 3-5mg for simple powders). Small sample pans (0.1ml) of inert or treated metals (al, pt, stainless). Several pan configurations, e.g., open, pinhole, or hermetically- sealed pan. Same material and configuration should be used for the sample and the reference. Material should completely cover the bottom of the pan to ensure good thermal contact. Avoid overfilling the pan to minimize thermal lag from the bulk of the material to the sensor aluminum, platinum, Ni, Cu, quartz (Figure 16).
Figure 16: Sample preparation.

Purge gases: Sample may react with air-oxidising or burning. Control moisture content of atmosphere control atmosphere. Use inert gas e.g. Nitrogen or argon use argon. Flowing purge gas flowing gas. In some cases deliberately choose reactive gas, e.g. hydrogen to reduce an oxide to metal, carbon dioxide which affects decomposition of metal carbonate. Removes waste products from sublimation or decompositionde composition.
Best practices for thermal analysis: Proper instrument calibration. Use purge gas (n2 or he) to remove corrosive off-gases. Small sample size. Good thermal contact between the sample and the temperature- sensing device. Proper sample encapsulation. Start temperature well below expected transition temperature. Slow scanning speeds (unless aiming to obscure thermal transitions, e.g. fast scan DSC). Avoid decomposition in the DSC (Run TGA first -it's easier to clean up!) [1-15](Table 5 & 6).
Figure 17: DTA.
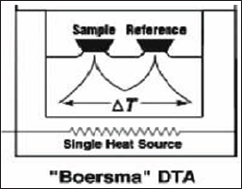
Table 5: Difference between DSC & DTA.
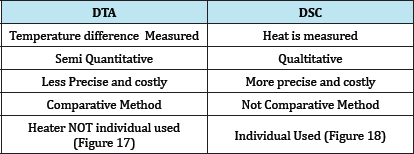
Figure 18: DSC.
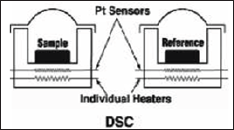
Table 6: Thermal analysis techniques.
References
- Kasture AV (2010) Pharmaceutical Analysis Vol-II. In: (9th, 10th edn), Nirali prakashan, India, pp. 258-263.
- Mackenzie RC (1979) Thermochim acta 28(1).
- Freizer H. Reactive groups as reagents: introduction and inorganic applications. In: Treatise on Analytical.
- Kolthoff IM, Elving PJ (1983) Chemistry. In: (2nd edn), Interscience Pubishers, USA.
- Earnest CM (1984) Modern Thermogravimetry. In: Anal Chem 56(13): 1471A-1486A.
- Dorsey DL, Buecker B (1986) TGA Identifies Scrubber Materials Research and Development 28: 93.
- Day RA, Underwood AL (1993) Quantitative Analysis. In: (6th edn), Prentice Hall of India Pvt. Ltd, India.
- Hill JO (1991) For better thermal analysis and calorimetry. In: (3rd edn), International Confederation for Thermal Analysis, pp. 1-93.
- Atkins PW (1994) Physical Chemistry. In: (5th edn), W H Freeman & Co, USA.
- Kealtch CJ, Doltimore D (1975 ) An Introduction to Thermogravimetry. In: (2nd edn), London, pp 1-164.
- Ashtosh K (2006) Pharmaceutical drug analysis. In: New age international publisher, India, pp. 193-202.
- Douglas AS (1980) Principle of instrumental analysis. In: (2nd edn), Saunders College Publications, USA, pp. 654-662.
- www.wikipedia.org
- www.authorstream.com
- www.slideshare.com
© 2017 Nirav Soni. This is an open access article distributed under the terms of the Creative Commons Attribution License , which permits unrestricted use, distribution, and build upon your work non-commercially.
 a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in
a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in 







.jpg)






























 Editorial Board Registrations
Editorial Board Registrations Submit your Article
Submit your Article Refer a Friend
Refer a Friend Advertise With Us
Advertise With Us
.jpg)






.jpg)














.bmp)
.jpg)
.png)
.jpg)










.jpg)






.png)

.png)



.png)






