- Submissions

Full Text
Modern Applications in Pharmacy & Pharmacology
Potential Application of Raman Micro-Spectroscopy as an In vitro Drug Screening and Companion Diagnostic Tool for Clinical Application: Chemotherapeutic Drug Mechanism of Action, Cellular Effects and Resistance
Z Farhane1,2* F Bonnier3 and H J Byrne1
1FOCAS Research Institute, Dublin Institute of Technology, Ireland
2School of Physics and clinical and optometric Sciences, Dublin Institute of Technology, Ireland
3Université François Rabelais de Tours, France
*Corresponding author: Z Farhane, FOCAS Research Institute, School of Physics and clinical and optometric Sciences, Dublin Institute of Technology, Kevin Street, Dublin 8, Ireland
Submission: October 31, 2017; Published: November 13, 2017

ISSN 2637-7756Volume1 Issue2
Abstract
To be considered as an in vitro companion diagnostics technique to screen for personalised therapies, Raman micro-spectroscopy should be able to monitor sub cellular interaction with chemotherapeutic drugs and to characterise cellular resistance. Investigations demonstrate the ability of Raman micro-spectroscopy not only to track the sub cellular accumulation of the drug as a function of time, but also to identify its mechanism of action, the subsequent cellular response and to identify cellular resistance. Despite the fact that different cell lines show different chemotherapeutic resistance, the chemical binding signature appears to be identical foranti-cancer drugs which belong to the same chemotherapeutic group, with implications of different mechanisms of action as a function of time and dose.
Keywords: Raman micro-spectroscopy; Chemotherapeutic drugs; Mechanism of action; Cellular effects; Resistance; Companion diagnostic
Introduction
Traditional diagnostic methods are largely based on identification of morphological changes in cells or tissues, rather than analysis of the underlying biochemistry, and so are subjective and prone to error. Therefore, there is a need to develop more effective non-invasive diagnostic and prognostic techniques for a single clinical investigation, either in real time diagnosis or an in vitro way which could be applied to in vivo situations. An increased emphasis on in vitro techniques for evaluation of drug mechanisms and efficacies has also emerged from the introduction of European and US legislation which restrict the use ofanimal models in cosmetic and pharmaceutical development (EU Directive-2010/63/EU and US Public Law 106-545, 2010, 106th Congress), adding further to the demand for novel biological screening methodologies.
As an alternative, Raman micro-spectroscopy, a vibrational spectroscopy, is an optical technique based on inelastic scattering of light by vibrating molecules, demonstrated by C.V. Raman in 1928 [1-4]. It is based on the interaction of photons with the vibrational states of the molecules in the sample, causing them to scatter in elastically, giving rise to the "Raman effect". As the vibrations are molecularly specific, the technique can provide chemical fingerprints of complex biological samples.
Figure 1: Visible image of human lung adenocarcinoma cell line A549.
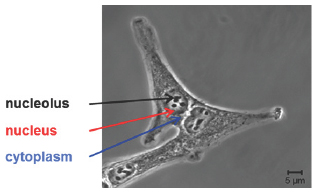
As an in vitro molecular fingerprinting technique with optical resolution, Raman micro-spectroscopy is able to monitor biochemical processes, drug uptake, efficacy and mode of action and mechanisms of interaction of chemotherapeutic drugs at a sub cellular level and therefore, can help guide drug design and discovery, and potentially even evaluate signatures of drug resistance, towards potential applications in personalised therapy and as a companion diagnostic tool (Figure 1).
To this end, different lung cell lines were used in order to explore the potential of Raman micro-spectroscopy to elucidate drug pathways, chemical binding signatures, mechanisms of action and efficacy, and physiological cellular responses to the drug exposure. Spectra were taken from the three cellular compartments, namely nucleolus, nucleus and cytoplasm, as shown in Figure 1. As chemotherapeutic agents, Doxorubicin (DOX) and Actinomycin D (ACT), both anthracyclines widely used clinically, especially for lung cancer, were employed as pilot molecules.
Figure 2: Raman spectra of A. Nucleolus, B. Nucleus and C. Cytoplasm of A549 cell line.
Highlighted regions correspond to DNA/RNA and lipids features.

In Figure 2, typical Raman cellular spectra, with specific features related to cellular components, for example DNA, RNA and lipids, are shown.
Raman Micro-Spectroscopy for Chemotherapeutic Screening and Cellular Resistance
Many studies have reported the use of vibrational spectroscopy to monitor the effects of anticancer agents on cancer cells, including, polyphenols [5] cardiotonic steroids [6], platinum compounds [7], epidermal growth factor inhibitor [8], gold based metallo drugs [9], plant alkaloids [10-13] and anthracyclines [14-18].
Using DOX, Raman micro-spectroscopy clearly demonstrates that it accumulates and saturates first the nucleoli, selectively targeting the RNA, then the nucleus, before it accumulates in the cytoplasm, after nuclear disruption [19,20].
Raman micro-spectroscopy can also differentiate the biochemical responses associated with the sub cellular regions of nucleolus, nucleus and cytoplasm, both in terms of the mechanisms of action (DNA intercalation in the nuclear area and ROS production in cytoplasmic region), and the subsequent cellular metabolic responses for the same cell lines and between different cell lines with difference in resistance, evidenced by an increase in protein features, related to expression of anti-apoptotic proteins and tolerance to DNA damage and implication of DNA repair mechanisms manifest as an increase in DNA signatures [15,20]. A similar response profile was observed for ACT in the same cell lines, both in terms of time evolution and cellular pathways, which suggests that the anthracycline chemotherapeutic group targets the nucleolus first, binding with RNA, and nucleus second, binding with DNA, before accumulating in the cytoplasm, and spectroscopic signatures of the mechanism of action, by DNA intercalation, despite the fact that ACT exhibits higher toxicity then DOX [ref]. So, Raman micro-spectroscopy has shed further light on the current understanding of the mode of action of the anthracyclines which is considered to interact only with nuclear DNA in parallel with cytoplasm [21-23].
Moreover, the studies of Cisplatin and Vincristine demonstrate that a drug can have different modes of action, dependent on dose [11,24]. In fact, Cisplatin, an alkylating agent which binds with DNA forming inter and intra strand crosslink's, and Vincristine, an alkaloid which binds to microtubules, appear to intercalate into DNA at high doses.
Conclusion
The ability of Raman micro-spectroscopy not only to track the kinetics and accumulation of chemotherapeutic drugs, in vitro, at a sub cellular level, but also to identify their different mechanisms of action according to different time points and doses and to identify cellular resistances, opens up potential clinical applications as a Companion Diagnostics tool, and ultimately personalised medicine approaches as a predictive tool for patient responses in individualised treatment.
References
- Paudel A, Raijada D, Rantanen J (2015) Raman spectroscopy in pharmaceutical product design. Adv Drug Deliv Rev 89: 3-20.
- Gala U, Chauhan H (2015) Principles and applications of Raman spectroscopy in pharmaceutical drug discovery and development. Expert Opin Drug Discov 10(2): 187-206.
- Byrne HJ, Ostrowska KM, Nawaz H, Dorney J, Meade AD, et al. (2014) Vibrational spectroscopy: disease diagnostics and beyond, in optical spectroscopy and computational methods in biology and medicine. In: Baranska M (Ed.), Springer, Netherlands, pp. 355-399.
- Smith GP, McGoverin CM, Fraser SJ, Gordon KC (2015) Raman imaging of drug delivery systems. Adv Drug Deliv Rev 89: 21-41.
- Derenne A, Van Hemelryck V, Lamoral Theys D, Kiss R, Goormaghtigh E (2013) FTIR spectroscopy: A new valuable tool to classify the effects of polyphenolic compounds on cancer cells. Biochim Biophys Acta (BBA) - Molecular Basis of Disease 1832(1): 46-56.
- Gasper R, Mijatovic T, Benard A, Derenne A, Kiss R, et al. (2010) Biochim Biophys Acta 1802: 1087-1094.
- Nie F, Yu XL, Wang XG, Tang YF, Wang LL, et al. (2010) Down-regulation of CacyBP is associated with poor prognosis and the effects on COX-2 expression in breast cancer. Int J Oncol 37(5): 1261-1269.
- Mashtoly SF El, Petersen D, Yosef HK, Mosig A, Reinacher Schick A, et al. (2014) Label-free imaging of drug distribution and metabolism in colon cancer cells by Raman microscopy. Analyst 139(5): 1155-1161.
- Roux K le, Prinsloo LC, Meyer D (2014) Metallodrug induced apoptotic cell death and survival attempts are characterizable by Raman spectroscopy. Applied Physics Letters 105(12): 123702.
- Hartmann K, Becker Putsche M, Bocklitz T, Pachmann K, Niendorf A, et al. (2012) A study of Docetaxel-induced effects in MCF-7 cells by means of Raman microspectroscopy. Analytical and Bioanalytical Chemistry 403(3): 745-753.
- Nawaz H, Garcia HA, Meade AD, Lyng FM, Byrne HJ (2013) Raman micro spectroscopy study of the interaction of vincristine with A549 cells supported by expression analysis of bcl-2 protein. Analyst 138(20): 6177-6184.
- Salehi H, Middendorp E, Panayotov I, Collart Dutilleul PY, Vegh AG, et al. (2013) Confocal Raman data analysis enables identifying apoptosis of MCF-7 cells caused by anticancer drug paclitaxel. J Biomed Opt 18(5): 56010.
- Salehi H, Derely L, Vegh AG, Durand JC, Gergely C, et al. (2013) Label-free detection of anticancer drug paclitaxel in living cells by confocal Raman microscopy. Applied Physics Letters 102(11): 113701.
- Moritz TJ, Taylor DS, Krol DM, Fritch J, Chan JW (2010) Detection of doxorubicin-induced apoptosis of leukemic T-lymphocytes by laser tweezers Raman spectroscopy. Biomed Opt Express 1(4): 1138-1147.
- Farhane Z, Bonnier F, Casey A, Byrne HJ (2015) Raman micro spectroscopy for in vitro drug screening: subcellular localisation and interactions of doxorubicin. Analyst 140(12): 4212-4223.
- Farhane Z, Bonnier F, Byrne HJ (2017) An in vitro study of the interaction of the chemotherapeutic drug Actinomycin D with lung cancer cell lines using Raman micro-spectroscopy. J Biophotonics.
- Schie IW, Alber L, Gryshuk AL, Chan JW (2014) Investigating drug induced changes in single, living lymphocytes based on Raman microspectroscopy. Analyst 139(11): 2726-2733.
- Guo J, Cai W, Du B, Qian M, Sun Z (2009) Raman spectroscopic investigation on the interaction of malignant hepatocytes with doxorubicin. Biophys Chem 140(1-3): 57-61.
- Farhane Z, Bonnier F, Byrne HJ (2017) Monitoring doxorubicin cellular uptake and trafficking using in vitro Raman microspectroscopy: short and long time exposure effects on lung cancer cell lines. Analytical and Bioanalytical Chemistry 409(5): 1333-1346.
- Farhane Z, Bonnier F, Howe O, Casey A, Byrne HJ (2017) Doxorubicin kinetics and effects on lung cancer cell lines using in vitro Raman microspectroscopy: binding signatures, drug resistance and DNA repair. J Biophotonics.
- Rabbani A, Finn RM, Ausio J (2005) The anthracycline antibiotics: antitumor drugs that alter chromatin structure. Bioessays 27(1): 50-56.
- Minotti G, Menna P, Salvatorelli E, Cairo G, Gianni L (2004) Anthracyclines: molecular advances and pharmacologic developments in antitumor activity and cardiotoxicity. Pharmacol Rev 56(2): 185-229.
- Yaqub F (2013) The Lancet Oncology 14: e296.
- Nawaz H, Bonnier F, Meade AD, Lyng FM, Byrne HJ (2011) Comparison of subcellular responses for the evaluation and prediction of the chemotherapeutic response to cisplatin in lung adenocarcinoma using Raman spectroscopy. Analyst 136(12): 2450-2463.
© 2017 Z Farhane, et al. This is an open access article distributed under the terms of the Creative Commons Attribution License , which permits unrestricted use, distribution, and build upon your work non-commercially.
 a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in
a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in 







.jpg)






























 Editorial Board Registrations
Editorial Board Registrations Submit your Article
Submit your Article Refer a Friend
Refer a Friend Advertise With Us
Advertise With Us
.jpg)






.jpg)














.bmp)
.jpg)
.png)
.jpg)










.jpg)






.png)

.png)



.png)






