- Submissions

Full Text
Modern Approaches in Drug Designing
Metal Ions, Ligands and Complexes in Medicinal Chemistry
Jagessar RC*
Department of Chemistry, Faculty of Natural Sciences, University of Guyana, South America
*Corresponding author: Jagessar RC, Department of Chemistry, Faculty of Natural Sciences, University of Guyana, South America
Submission: January 09, 2023;Published: January 26, 2023

ISSN: 2576-9170 Volume4 Issue1
Abstract
Metal ions, ligands and complexes have shown significant inhibitory activities against human ailments such as cancer, diabetes, bacterial and fungal infections. Good activity has been predominantly restricted to transition metal ions and complexes. It’s important that ligands are properly designed to have maximum activity on disease receptor cells. Furthermore, Structure Activity Relationship (SAR) of ligands and complexes can be modulated to ensure maximum activity against infected cells. However, synthetic drugs of ligands and metal complexes suffer from their side effects on receptors cells, toxicity and in some cases ardous synthesis and less environmental compatibility. This paper gives an overview of some current trends in ligands and metal complexes design and activity on human receptor cells.
Keywords: Metal ions; Ligands; Complexes; Cancer; Diabetes; Bacterial; Fungal effects; Structure activity relationship
Introduction
Figure 1: Anti-cancer agents.
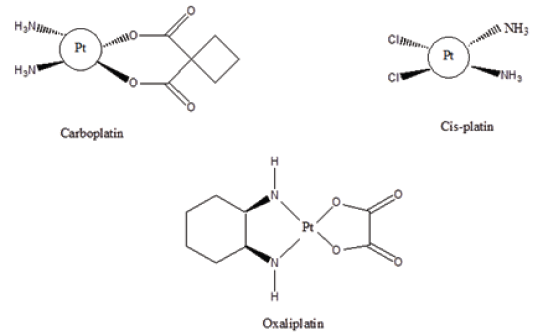
Ligands are neutral or anionic molecule that donate a pair of electrons to a vacant metal orbital [1-2]. Ligands can be monodentate, bidentate, tridentate. The number of atoms of a ligand that is coordinated to a metal ion is referred to as its dentary. The union of a ligand and a metal orbital (p, d or f) will result in the formation of a complex of varying colors (Figure 1). These complexes can be diamagnetic (align with the magnetic field) or can be paramagnetic (oppose the applied magnetic field). Ligands can be high spin i.e., having the maximum number of unpair electrons or can be low spin, having the maximum number of paired electrons. Complexes come in a variety of shapes. These include planar, trigonal planar, square planar, trigonal bipyramidal [1]. The shape of ligands and complexes has influenced their interactions with receptor cells and thus mechanism of action. Chelator design can affect kinetic properties, catalytic mechanisms and donor interactions [2]. The Periodic Table consists of over 103 elements which mean that complexes are too numerous to mention (Figure 2). The union of a metal ion and a ligand is called a complex or a chelate. Metal ions, ligands and complexes have played important roles in medicinal and Pharmaceutical chemistry [3-6]. This paper outlines the application of Ligands and Complexes in medicinal and Pharmaceutical chemistry (Table 1).
Figure 2:Structure of budotitane.
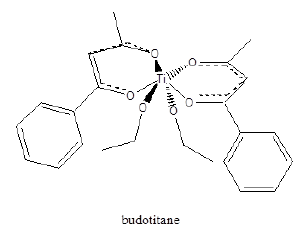
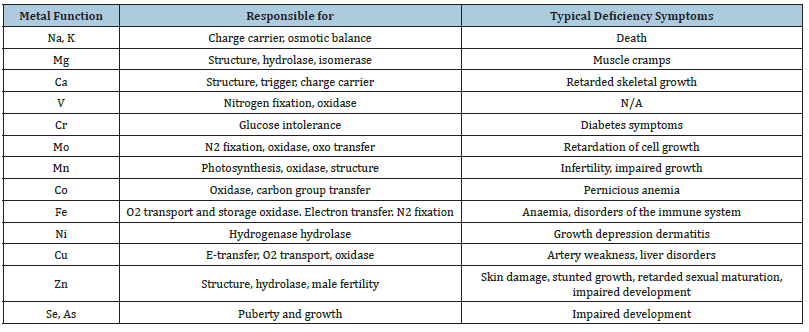
Table 1:Functions of metal ions in medicine and Pharmacy.
Application of Ligands in Pharmaceutical and Medicinal Chemistry
Metal ions
Metal cations are present in the biological systems as chelates or coordination complexes [7]. Metal ions have diverse roles in the human biological system. These include calcium role in enamel and toothpaste, iron in blood and muscle function, molybdenum in the metabolism of purine, zinc in insulin and various enzymatic functions, Also, chromium in the metabolism of glucose and magnesium in the transmission of nerve impulses [8].
Metal complexes
Figure 3: Structure of a heteronuclear Ru-Cu terpyridine complex for DNA binding (Ru) and DNA cleavage (Cu).
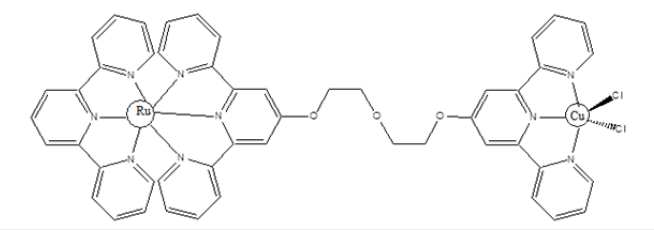
Metal complexes have been used in the treatment of neurodegenerative diseases. These include their use in the treatment of Alzheimer’s disease (Figure 3). Chelating agents such as clioquinol and elesclomol have been used in the treatment of Alzheimer’s disease. Cis-Platin have been used for the treatment of testicular and ovarian cancer since 1978. Cis-platin kills cancer cells via cross-linking with the DNA strand and inhibition of transcription phenomenon [9-11]. Despite its curative purposes, Cis-platin is not effective as a Universal anti-cancer agent. This has led to the synthesis of other platinum compounds as anticancer agents. These include carboplatin, oxaliplatin, lobaplatin, heptaplatin etc. [12-13].
Figure 4:Structure of an Au-clotrimazole antiparasitic combination agent.
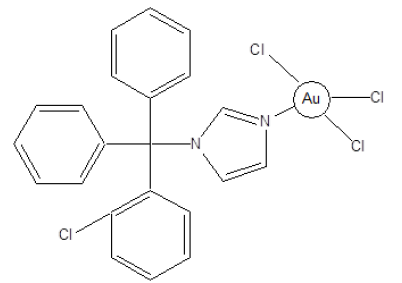
Titanium (IV) complexes such as badotitane, which is a β-diketonato complexes of titanium and titanium dichloride have shown antitumor activity in human clinical trials with low toxicity levels. However, these complexes are not stable [14]. Metal complexes have been used as combination agents to form a single compound, capable of modulating multiple targets simultaneously. For example, the combination of a DNA targeting agent with a DNA cleaving agent is shown in Figure 3 below. The DNA-cleavage activity of the redox-active Cu-terpyridine unit was significantly increased when attached to the Ru metal intercalator [15]. The ligation of anti-parasitic agent such as clotrimazole and ketoconazole with transition metals such as Cu2+ and Au2+ improves their activity against Trypanosoma cruzi (Chagas disease). It’s the imidazole moiety of these drugs that coordinate to the transition metal ions (Figure 4).
Vanadium ions such as vanadyl (VO2+) or vandate (VO43-) enhances the effects of insulin and have been studied as antidiabetic agents [15]. On a similar note, bifunctional vanadium complexes have been developed using bioactive ligands based on metformin, thiazolidinediones and curcumin. Figure 5 shows the structure of a vanadium-thiazolidinedione coordination agent for diabetes therapy. Lanthanide coordination complexes show considerable emissive optical properties, exhibiting sharp metalbased emission bands and long luminescence lifetimes. As such they have been used as Magnetic Resonance Imaging agents. Lanthanide emission bands are sharp and are characteristic for each metal. The most common Ln3+ ions for optical imaging purposes are Eu3+ and Tb3+, as these ions exhibit optimal emissive properties [16-18] Luminescent Ru2+ complexes have been extensively studied as DNA binding agents and as cell imaging probes [19-20].
Figure 5: Structure of a vanadium-thiazolidinedione combination agent for diabetes therapy.

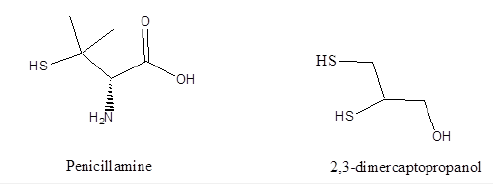
Ligand design is of crucial importance in their use in preventing metal ion overload, either from ingestion or a breakdown in the uptake, transport, or storage of essential metal ions, significant toxicity [21-22]. Toxic metal poisoning and metal overload disorders such as Wilson’s disease (Cu) and Hemochromatosis (Fe) are treated with metal ion chelators such as penicillamine, EDTA and deferoxamine [23-24], Figure 6. Metal ion dysregulation has been implicated in neurodegenerative diseases such as Parkinson’s disease, Alzheimer’s Disease (AD), Creutzfeldt-Jakob disease and Amyotropic Lateral Sclerosis [25]. 2,3-Dimercaptopropanol known as British Anti-Lewsite (BAL) is used as an antidote to Lewisite (dichlorovinylase). The first use of BAL was associated with arsenic therapies against syphilis and industrial accidents with arsenic [26].
Figure 6:Metal ion chelators.
Lanthanide coordination complexes show considerable emissive optical properties, exhibiting sharp metal-based emission bands and long luminescence lifetimes. As such they have been used as Magnetic Resonance Imaging agents. Lanthanide emission bands are sharp and are characteristic for each metal. The most common Ln3+ ions for optical imaging purposes are Eu3+ and Tb3+, as these ions exhibit optimal emissive properties [16-18] Luminescent Ru2+ complexes have been extensively studied as DNA binding agents and as cell imaging probes [19-20]./
Ligand design is of crucial importance in their use in preventing metal ion overload, either from ingestion or a breakdown in the uptake, transport, or storage of essential metal ions, significant toxicity [21-22]. Toxic metal poisoning and metal overload disorders such as Wilson’s disease (Cu) and Hemochromatosis (Fe) are treated with metal ion chelators such as penicillamine, EDTA and desferrioxamine [23-24], Figure 6 Metal ion dysregulation has been implicated in neurodegenerative diseases such as Parkinson’s disease, Alzheimer’s Disease (AD), Creutzfeldt-Jakob disease and Amyotropic Lateral Sclerosis [25]. 2,3-Dimercaptopropanol known as British Anti-Lewsite (BAL) is used as an antidote to Lewisite (dichlorovinylase). The first use of BAL was associated with arsenic therapies against syphilis and industrial accidents with arsenic [26].
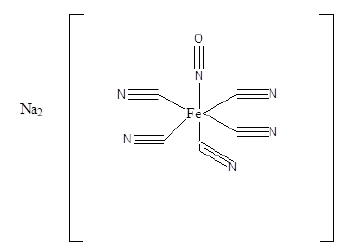
Metal complexes of iron, gold, ruthenium, cobalt, rhodium, copper, cobalt, zinc, osmium and palladium have shown effective anti-malarial activity. The zinc (II) complex of amodiaquine, Figure 7 exhibited anti-malarial activity towards Plasmodium berghei [27]. Ligands of o-vanillin-(4-methylthiosemicarbazone) with Ga (III), Fe (III) and (III) have been shown to be active against malaria [28]. Ferrocenyl carbohydrate, Figure 8 have shown potential as an anti-malarial compound [29-30]. Sodium nitroprusside, Figure 9 interacts with a drug via its sulfhydryl groups to deliver nitric oxide. The latter causes fast vasodilation and acute lowering of the blood pressure [31-32].
Figure 7:Structure of zinc (II) with amodiaquine ligand
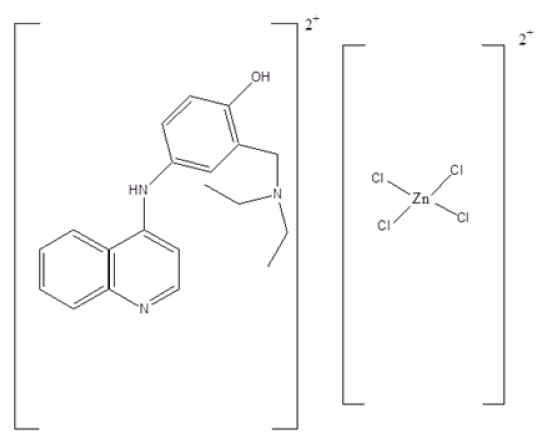
Figure 8:Ferrocenyl carbohydrate conjugate.
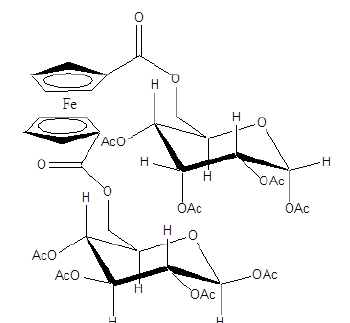
Figure 9:Structure of sodium nitroprusside.
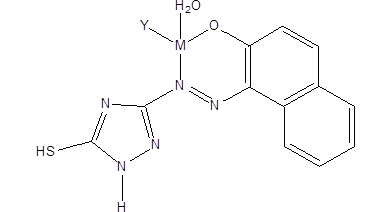
Many azo dyes and coordination compound of azo dye inhibit the growth of bacteria and fungi. The chelate ligand of 1[(5-mercapto- 1-H1,2,4-triazole-3-y1) diazenyl]-naphthalen2-ol with metals of Mn2+, Co2+, Ni2+ and Cu2+, Figure 10 have shown potency against the growth of E. coli, S. aureus, A. flavus and C. albicans29.
Figure 10:Structure of Mn (II), Co (II), Na(II) and Cu (II) complexes.
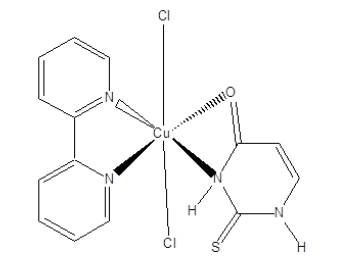
Complex [CuCl2(bipy)(L)], Figure 11 has shown anti-fungal activity against Candida albicans and thus exhibited fungicidal activity against planktonic and sessile cells.
Figure 11:Coordination compound of Cu (II).
Conclusion
Metal ions, ligands and metal complexes play important roles in medicine and pharmacy. These include antimicrobial, antidiabetic, anticancer etc. activities. It’s important that ligands are properly designed to chelate metal ions to form complexes and of the best fit to interact with receptor cells. The same also goes for metal complexes or chelates. Ligands are effective in preventing metal ion overload. These include penicillamine, EDTA and desferrioxamine. Metal complexes activity can be augmented via a combination of other metal complexes bearing different hetero nuclear centers. Medicinal ligands can further increase their activity via coordination to selective metal centers.
References
References
- Bell CF, Lott KAK (1966) Modern approach to Inorganic Chemistry. In: D Van (Ed.), (2nd edn) Nostrand Company, INC. Princeton, New Jersey, USA.
- Spencer J, Walden B (2017) Special focus: Metals in medicine. Future Med Chem 10(6): 607-609.
- Martin J, Ales MR, Asuero AG (2018) An overview on ligands of therapeutically interest. Pharmacy & Pharmacology International Journal 6(3): 198-214.
- Barnham KI, Bush A (2014) Biological metals and metal-targeting compounds in major neurodegenerative diseases. Chem. Soc Rev 43 (19): 6727-6749.
- Bass A (2015) Metals in medicine: An overview. Sci Revs Chem Commun 5(2): 77-87.
- Dabroniak JC (2017) Metals in medicine. (2nd edn), Wiley, New York, USA.
- Virag I, Erdodi F, Gergely P (2016) Bioinorganic chemistry for medicinal students. Medicinal Chemistry, Hungary, p. 104.
- Ferguson LN (1981) Chelates in chemotherapy. Ed Chem 18: 166-168.
- Ronconi I, Fregona D (2009) The midas touch in cancer chemotherapy: From platinum-to gold-dithiocarbamato complexes. Dalton Trans 48: 10670-10680.
- Wilson II, Lippard SI (2014) Synthetic method for the preparation of platinum anticancer complexes. Chem Rev 114(8): 470-4495.
- Jia O, Ouyag R, Cao P, Xiao T, Xia Z, et.al. (2017) Review: Recent advances and future development of metal complexes as anticancer agents. J Coord Chem 70(13): 2175-2201.
- Ndagi U, Mhlongo N, Soliman ME (2017) Metal complexes in cancer therapy-an update from drug design perspective. Drug Des Devel Ther 3(11): 599-616.
- Medici S, Peana M, Nurchi VM, Lachowicz JI, Crisponi G, et.al. (2015) Noble metals in medicine: Latest advances. Coord Chem Rev 284: 329-350.
- Buettner KM, Valentine AM (2012) Bioinorganic chemistry of titanium. Chemical Reviews 112(3): 1863-1881.
- Chiang L, Jones MR, Ferreira CL, Storr T (2012) Multifunctional ligands in medicinal inorganic chemistry-current trends and future directions. Curr Top Med Chem 12(3): 122-144.
- Montgomery CP, Murray BS, New EJ, Pal R, Parker D (2009) Cell penetrating metal complex optical probes. Targeted and responsive systems based on lathanide luminescence. Acc Chem Res 42(7): 925-937.
- New EJ, Parker D, Smith DG, Walton JW (2010) Development of responsive lathanide probes for cellular applications. Curr Opin Chem Biol 14(2): 238-246.
- Demeunynck MC, Bailly WD (2002) Small molecule DNA and RNA binders: From synthesis to nuclei acid complexes, Wiley VCH, Weinheim, Germany.
- Zeglis BM, Pierre VC, Barton JK (2007) Metallo-intercalators and metallo-insertors. Chem Commun (44): 4565-4579.
- Flora SJ (2009) Metal poisoning: Threat and management. Al Ameen J Med Sci 2(2): 4-26.
- Hengstler, JG, Bolt HM (2008) A special issue on metal toxicity. Arch Toxicol 82: 489-491.
- Brewer GJ (2009) Zinc and tetrathiomolybdate for the treatment of Wilson’s disease and the potential efficacy of anti-copper therapy in a wide variety of diseases. Metallomics 1(3) 199-206.
- Nielsen P, Fischer R, Buggisch P, Janka Saub G (2003) Effective treatment of hereditary haemochromatosis with desferrioxamine in selected cases. Br J Haematol 123(5): 952-953.
- Barnham KJ, Masters CL, Bush AI (2004) Neurodegenerative disease and oxidative stress. Nat Rev Drug Discovery 3: 205-214.
- Scott IE, Orvig C (2009) Medicinal inorganic chemistry approaches to passivation and removal of aberrant metal ions in disease. Chem Rev 109(10): 4885-4810.
- Mohamed HS, Tripathi VD (2020) Medicinal applications of coordination complexes. 1st International virtual conference on pure science. Journal of Physics: Conference Series.
- Rafique S, Idrees M, Nasim A, Akbar, H & Athar A (2010) Transition metal complexes as potential therapeutic agents. Biotechnology and Molecular Biology Reviews 5(2): 38-45.
- Herrmann C, Salas PF, Cawthray JF, Kock C, Patrick BO, et al. (2012) 1,1-disubstituted ferrocenyl carbohydrate chloroquine conjugates as potential antimalarial drug-thoughts on the mechanism of action. Molecules 13: 2900-2907.
- Ferreira CL, Ewart CB, Barta CA, Little S, Yardley V, et al. (2006) Synthesis, structure, and biological activity of ferrocenyl carbohydrate conjugates. Inorg Chem 45(20): 8414-8422.
- Hottinger DG, Beebe DS, Kozhimannil T, Prielipp RC, Belani KG (2014) Sodium nitroprusside in 2014: Clinical concepts review. J Anaesthesiol Clin Pharmacol 30(4): 462-471.
- Villarreal EG, Flores S, Kriz C, Iranpour N, Bronicki, RA, et al. (2020) Sodium nitroprusside versus nicardine for hypertension management after surgery: A systematic review and meta-analysis. J Card Surg 35(5): 1021-1028.
- Mohammed HS, Tripathi VD, Darghouth AA (2019) Synthesis, characterization, DFT calculation and antimicrobial activity of Co (II) and Cu (II) complexes with azo dye. In Journal of Physics: Conference Series 1294(5): 1-9.
- Ghamry HA, Fathalla SK, Gaber M (2018) Synthesis, structural characterization, and molecular modeling of bidentate azo dye metal complexes: DNA interaction to antimicrobial and anti-cancer activities. Applied Organometallic Chemistry 32(3): e4136.
- Gomes DF, Araujo AA, Pires DR, Regiane VL, Fukuda P, et al. (2018) A promising Copper (II) complex as antifungal and antibiofilm drug against yeast infection. Molecules 23(8): 1856.
© 2023 Jagessar RC. This is an open access article distributed under the terms of the Creative Commons Attribution License , which permits unrestricted use, distribution, and build upon your work non-commercially.
 a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in
a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in 







.jpg)






























 Editorial Board Registrations
Editorial Board Registrations Submit your Article
Submit your Article Refer a Friend
Refer a Friend Advertise With Us
Advertise With Us
.jpg)






.jpg)














.bmp)
.jpg)
.png)
.jpg)










.jpg)






.png)

.png)



.png)






