- Submissions

Full Text
Modern Approaches in Drug Designing
Formulations and Evaluation of Vernonia Amygdalina and Gongronema Latifolium Extract Capsules in Streptozotocin-Induced Diabetic Rats
Michael Adikwu*
Department of Pharmaceutics, Faculty of Pharmaceutical Sciences, University of Nigeria, Nigeria
*Corresponding author: Michael Adikwu, Department of Pharmaceutics, Faculty of Pharmaceutical Sciences, University of Nigeria, Nigeria
Submission: May 09, 2022;Published: September 09, 2022

ISSN: 2576-9170 Volume4 Issue1
Abstract
Recently, there has been great interest in herbal medicines for treatment of diverse diseases. Earlier studies have been done on Vernonia Amygdalina (VA) and Gongronema Latifolium (GL) for antidiabetic effect using various animal models. This study focused on the formulation and evaluation of capsules of VA and GL. Their combinations with standard antidiabetic drug glibenclamide and metformin, were also formulated. The freeze-dried aqueous extracts and semi-purified VA and GL were used in formulation of capsules. Investigation on antidiabetic effects of capsules of plant extracts (individual and combined) on streptozotocin-induced diabetic rats was carried out. The maximum antidiabetic dose for capsules of aqueous extract for VA and GL were 150 and 800mg/kg respectively. The antidiabetic effect of aqueous extract of VA and GL capsules were 67.63+2.59 and 52+11.82 %, respectively. Combined VA and standard drug had no significant effect on antidiabetic activity (p=0.813) when compared with individual VA capsules. Combined VA and GL produced marked decrease in blood glucose of 87.52+2.48% (p<0.001). Based on the findings from investigations carried out, aqueous VA and GL combined capsules demonstrated significant antidiabetic activities. This research has now clearly demonstrated that the extracts can be formulated into standard dosage forms like capsules.
Introduction
There is a great variety of compounds that can be extracted and characterized from plants. One example is the harmaline, one of the indole alkaloids found in Peganiumharmalan (Zygophyllaceous) used in the treatment of dermatosis [1]. Another substance that can be found in plants is morphine from the opium puppy, which has high analgesic action and is still in use. Its molecule is used as a model to design new drugs [2]. The isolation of artemisinin highlighted the importance of investigating plants to produce new drugs [3]. Pacific yew, the original source of Taxol (an anticancer drug) is relatively new to the mainstream medicine. The plant, Curcuma long L has a wide application in medicine [4]. It can be used in the treatment of conditions such as inflammation, oxidative stress, nematode infestation, bacteria diseases, snake bite, hypertension, and liver disease. This work focused on formulation and evaluation of capsule solid dosage form of two antidiabetic herbs. Vernonia amygdalina, and Gongronema latifolium aqueous leaf extract capsules were formulated with an optimized formula. The leaf extracts were semi-purified individual and combined with standards drugs (glibenclamide and metformin) capsules were also formulated. Capsules are solid dosage forms in which the drug is enclosed within either a hard or soft soluble container or shell. The shells are usually formed from gelatin; however, they can also be made from starch or other suitable substances. Hard–shell capsule sizes vary from 000, the largest size that can be swallowed, to 5, which is the smallest. The different categories of capsules that exist include hard, soft, and modified-release capsules [5].
Materials and Method
Chemicals and reagents
Methanol, chloroform, hexane, petroleum ether, ethanol, ethylacetate (all from Sigma Aldrich, Co St Louis, USA), lactose (Santa Cruz USA), starch soluble (BDH, England), glycerin (Bestimunngsort, Germany), TLC silica gel 60 F254 (Merck, Darmstadt Germany), Silica gel (QualiChem’s, China), Chemical, China). Formaldehyde (M & B, England), Tween 20 solution (Sigma, Germany), diphenyl -2-picril hydroxyl (DPPH) (Sigma Aldrich, Germany). Distilled water, olive oil, normal saline (Juhel, Nigeria), Cholesterol Kits (Biosystems, Spain), other test kits (Randox Laboratories, U.K).
Preparation of methanol extract of VA and GL
A 400g quantity of VA and GL was extracted by cold maceration. The VA was immersed in aqueous methanol (80%) for 48h and shaken every 2h. It was filtered. Thereafter the filtrate was dried in rotary evaporator to obtain the Methanol Extract (ME).
Preparation of admixtures
The slurry of silica gel was prepared by adsorbing silica gel (100-200 mesh size) (20g) to the methanol extract of VA (5.00g) (1:4). A 10ml quantity of methanol was added to the extract and was dried in a hot air oven. The adsorbed extract was ground into a fine powder in a ceramic mortar with pestle. The powder was carefully layered on top of packed silica gel in column. Column chromatography separation was used to purify VA and GL with gradient solvent system (n- hexane, Chloroform, ethyl acetate and methanol) as the mobile phase.
Formulation of capsule dosage
The excipient (lactose) which was compatible with VA and GL was used as diluent. Talc was used as lubricant. The powder composed of drugs and excipients which were prepared by mixing in a glass mortar for 20min. The powder blend was filled into No 2 hard gelatin capsules (260mg) using a semiautomatic capsulefilling machine (Capsule CN, China).
Physic-chemical analysis of capsules
Physic-chemical analysis of all the capsules namely weight uniformity, disintegration and In-vitro dissolution tests were carried out.
Determination of effect of aqueous GL extract capsules on STZ - induced diabetic rat
Twenty –five rats of male sex weighing 120-150g were randomly divided into 5 groups (1-5) of five rats per group. The animals were fed with standard pellet diet and water ad libitum. The animals were fasted 14h before induction of diabetes by Intraperitoneal (IP) injection of a single dose of Streptozotocin (STZ) (65mg/kg body weight). The accurately weighed STZ was dissolved in distilled water and injected immediately within few min to avoid degradation. Diabetes was confirmed after 48h in rats that showed Fasting Blood Glucose (FBG) levels of >240mg/ dl and were selected for study. The animals in groups 1 and 5 were designated normal and diabetic control and received 2ml/kg of normal saline. The aqueous GL extract capsule was prepared and was orally administered at a dose of 800mg/kg to group 2 using an improvised method. Then glibenclamide (2mg/kg) and metformin (500mg/kg) were orally administered to group 3 and 4 respectively. Blood glucose of each rat was measured at 0, 1, 2, 4 and 8h using Açu-Check glucometer.
Dose- related anti-diabetic response of semi-purified VA capsules
Thirty- five rats of male sex weighing 120-150g were randomly divided into 7 groups (1-7) of five rats per group and fasted for 14h. Each rat in each group was intraperitoneally injected with accurately determined volume of STZ (65mg/kg) in distilled water. The animals were fed with standard pellet diet and water ad libitum for 48h. Diabetes was confirmed after 48h in rats that showed Fasting Blood Glucose (FBG) levels of >240mg/dl. The purified VA capsule accurately prepared was orally administered at doses of 50, 75 and 100mg/kg to groups 2-4 using an improvised method. Then standard glibenclamide (2mg/kg) and metformin (500 mg/ kg) was administered to groups 5 and 6. The control group (1) and diabetic group (7) received 2ml/kg of normal saline. Blood glucose of the treated rats was measured 0, 1, 2, 4 and 8h using Açu-check glucometer. The percentage glucose reduction was calculated using.
Measurement of biochemical parameters
These were measure according to standard methods. The blood was collected for serum enzyme and bilirubin assay. Those biochemical factors studied were aspartate Aminotransferase (AST), Alanine Aminotransferase (ALT), total bilirubin, total cholesterol, and total glycerides.
Result and Discussion
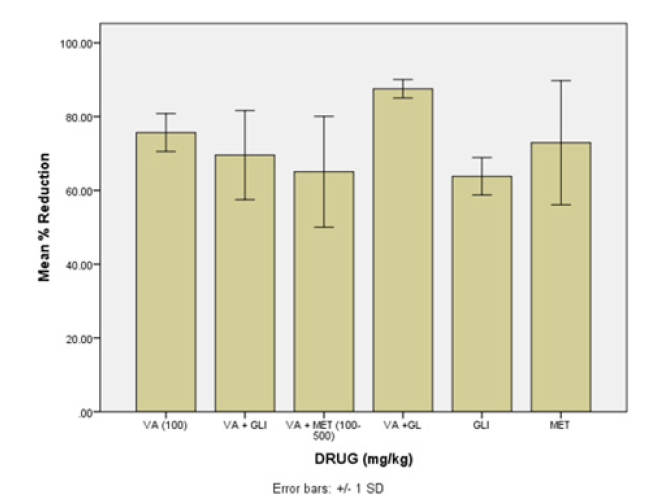
All capsules passed pharmacopeial requirements. The maximum antidiabetic dose of VA and GL aqueous leaf extract capsules were, 150 and 800mg/kg respectively. On the other hand, the maximum antidiabetic dose of semi- purified capsules of VA and GL were 100 and 400mg/kg respectively. Figures 1 & 2 show the drug release of VA and GL (individual and combined). The data showed sharp peaks of drug release profiles of VAPE combination with metformin or glibenclamide after 10min. Figure 3 shows antidiabetic effects of VA capsules individual and combined with standard drugs and GL. Combination of semi-purified VA and GL capsules had higher antidiabetic activity than individual drugs. However, combination of all purified drugs with standard capsules had no better activity against individual drugs. Assessment of toxicity and biochemical tests (AST, ALT, total bilirubin, total cholesterol, and total glycerides) of dosage forms confirmed they were safe. The decreased PCV and hemoglobin of the diabetic animals were remarkably improved in all the drugs capsules dosage forms. Furthermore, AST and ALT activities that were increased in diabetic control were reduced significantly in all the capsules dosage form. Conclusion. The objectives of the study were achieved, and it is expected that pharmaceutical companies will commercialize the manufacture of the capsules after further investigation. Further investigation on alternative formulation technique should be designed to improve antidiabetic activity of combinations with standard drugs to reduce the side effects minimally. It is also expected that further research to improve the capsule dosage forms with regards to stability, would benefit the growing number of producers.
Figure 1: Drug release of V. amygdalina and its combination with standard drugs. Blue: Vernonia amygdalina alone Red: Vernonia amygdalina plus Metformin Light Blue: Vernonia amygdalina plus Glibenclamide
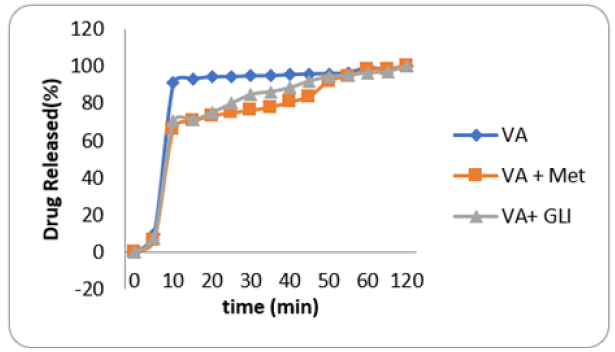
Figure 2: Drug release of Gongronema latifolium and its combination with standard drugs. Blue: Gongronema latifolium alone Red: Gongronema latifolium plus Metformin Light Blue: Gongronema latifolium plus Glibenclamide
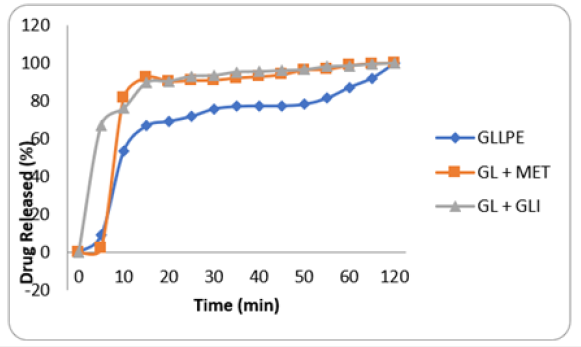
Figure 3: Combination effect of VA with standard drugs and GL on STZ- induced diabetic rat
Key.
VA= Veronica amygdalina alone
VA+GLI =Vernonia amygdalina plus Glibenclamide
VA+MET = Vernonia amygdalina plus Metformin
VA+GL = Vernonia amygdalina plus Gongrenema Latifo
GLI = Glibenclamide
MET = Metformin
References
- Iwu MM, Jackson JE, Schuster BG (1994) Medicinal plants in the fight against Leishmaniasis. Parasitology Today 10(2): 65-68.
- Phillipson JD (1994) Natural products as drugs. Society for Tropical Medicine and Hygiene 88(1): 17-19.
- Wang J, Xu C, Wong YK, Li Y, Liao F, et al. (2019) Artemisinin, the magic drug discovered from traditional Chinese medicine. Engineering 5(1): 32-39.
- Karlowicz-Bodalska K, Han S, Freier J, Smolenski M, Bodalska A (2017) Curcuma longa as medicinal herb in the treatment of diabetic complications. Acta Pol Pharm 74(2): 605-610.
- US Pharmacopoeia (2009) USP Pharmacist’ Pharmacopeia 1100-1105, 1108, 1096-1099.
© 2022 Michael Adikwu. This is an open access article distributed under the terms of the Creative Commons Attribution License , which permits unrestricted use, distribution, and build upon your work non-commercially.
 a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in
a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in 







.jpg)






























 Editorial Board Registrations
Editorial Board Registrations Submit your Article
Submit your Article Refer a Friend
Refer a Friend Advertise With Us
Advertise With Us
.jpg)






.jpg)














.bmp)
.jpg)
.png)
.jpg)










.jpg)






.png)

.png)



.png)






