- Submissions

Full Text
Modern Approaches in Drug Designing
“Trojan” RNA Viruses Carrying PAK18 for Therapy of PAK1-Dependent Diseases
Hiroshi Maruta*
PAK Research Center, Australia
*Corresponding author: Hiroshi Maruta, PAK Research Center, Australia
Submission: February 23, 2022;Published: September 08, 2022

ISSN: 2576-9170 Volume3 Issue5
Abstract
Around 1980, several viruses were found to carry an oncogenic mutant of cellular genes such as a Tyrkinase called SRC and a G protein called RAS, eventually causing malignant trans-formation in chicken and mouse. Reversely, if we can manage to insert into viral genomes several known anti-oncogenes such as NF1, NF2, p53, and RB, in principle, we could suppress the growth or metastasis of cancers. On the recent advent of COVID pandemics, a brand-new idea using this RNA virus as a carrier of anti-pathogenic genes such as PAK1-blockers, to reverse the pathogenic process, has suddenly emerged. Here as an example, I briefly introduce a PAK1-blocker called “PAK18”, a peptide of 18 amino acids derived from the major pathogenic kinase “PAK1”, which could suppress cancers and many other PAK1-dependent diseases by blocking the interaction of PAK1 with its activator called PIX. The Genetically Modified (GM) COVID carrying the mRNA coding PAK18 can infect lung and platelet cells through its spike proteins, but cannot cause either fibrosis or thrombosis, because these pathogenic processes require PAK1. Instead, this GM virus mass-produces PAK18 in target cells and suppresses a wide variety of PAK1-dependent diseases, including immune-suppression, boosting B/T cells to produce antibodies against COVID, thus working as a COVID vaccine as well, and eventually promoting the longevity.
Keywords: PAK1; PAK18; COVID; PIX; Cancer; Fibrosis; Thrombosis
Introduction
During last three decades, there are several attempts to use either Reovirus, a series of adeno-viral mutants or Genetically Modified (GM) viruses for the therapy of cancers and other diseases [1-3]. As infection of Reovirus depends on the oncogenic mutant of RAS, it would kill selectively RAS-cancers which represent more 30% of human cancers, more than 90% of deadly pancreatic cancers [1]. An adenoviral mutant called “Onix-015” selectively infects p53-deficient cancer cells which represent more than 50% of all human cancers [2]. Herpes Simplex Virus (HSV) requires TK (Thymidine Kinase) gene for its infection of cells. Thus, TK-deficient mutants of HSV (such as G207 mutant) can infect cancer cells in which TK is highly activated, while it cannot infect neuronal cells which no longer grow, due to low TK activity [3]. Unfortunately, however, none of these viruses or mutants has been used clinically for viro-therapy of cancers yet, if I understand correctly.
“GM” COVID-Based Therapy of Pak1-Dependent Diseases
The 2019 advent of COVID pandemics reminded us of the possibility of using a GM RNA virus carrying PAK1-blocking mRNAs encoding NF1, NF2, LKB1, and PTEN as well as PAK18, RAF87 and NF 91 or their mutants which block the oncogenic RAS-PAK1 signalling for the therapy of cancer and many other PAK1-Dependent Diseases (PDDs), which might be called viro-therapy of PDDs. These GM viruses such as GM-COVID can multiply themselves in host cells efficiently without causing any subsequent inflammation such as fibrosis and thrombosis, because these PAK1-blockers within GM viral RNA genome would suppress these PAK1-dependent phenotypes [4]; (Figure1). In an early 2022, when a variety of COVID mutants including “delta” and “omicron” have clearly un-powered a series of “old” COVID RNA vaccines against COVID-19, a unique idea suddenly ignited our mind for creating a “PAK18 variant” of COVID (called “Inspirin P”), which carries PAK18 (PIX-binding domain of PAK1) mRNA of AUG plus 54 bp plus UGA (just behind a strong promoter X) in its RNA genome (Figure 2). What is “PAK18”?
Figure 1: PAK1-dependent fibrosis by COVID.
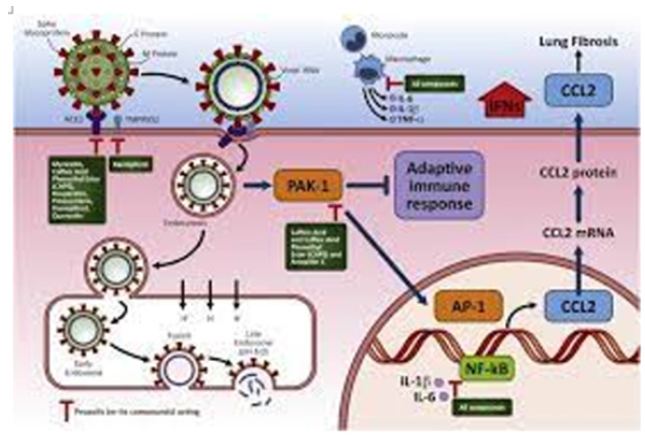
Figure 2:“Trojan” RNA viruses carrying “PAK18” for therapy of PAK1-dependent diseases.

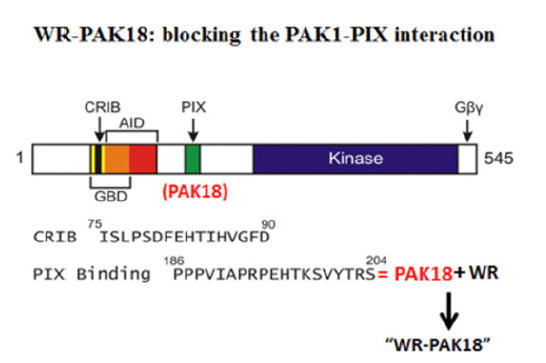
Back in 1994, Ed Manser’s team at Singapore National University (IMCB) cloned a new mammalian Ser/Thr kinase called “PAK1” (RAC/CDC42-activated kinase 1), which is very similar to myosin I heavy chain kinase isolated from a soil amoeba called Acanthamoeba [5,6]. A few years later, his team found a third activator of PAK1, called PIX, which is essential for the activation of PAK1 in cells [7]. PIX is an SH3 protein which binds a N-terminal Pro-rich domain of 18 amino acids (called “PAK18”) on PAK1 molecule [8]; (Figure 3). The peptide PAK18 blocks the PIX-PAK1 interaction, inhibiting PIX-dependent activation of PAK1 in RAS cancer cells and suppresses their actomyosin-based membraneruffling and malignant growth if PAK18 is microinjected [8]. Subsequently we developed a cell-permeable peptide called “WRPAK1” by attaching the cell-permeable (Trp/Arg-rich) peptide vector WR of 16 amino acids [9]. WR-PAK18 suppresses the RASinduced malignant transformation with IC50=5 micro-M, without any effect on normal cell growth [9]. However, WR could be easily digested by peptidases in our body, and therefore may not be so effective clinically, although it worked on a Schizophrenia model of mice at least.
Figure 3:WR-PAK18: blocking the PAK1-PIX interaction.
Instead, We Could Use Human RNA Viruses Such as Covid as an Effective Vector for PAK18
The spike protein of this virus is still able to bind ACE-2 of host lung cells, and penetrate through their plasma membranes, but cannot cause the PAK1-dependent fibrosis (inflammation of lungs), simply because PAK18 will block lung PAK1. Thus, this variant keeps infecting other host cells in the rest of our body, but without causing any PAK1-dependent diseases such as fibrosis, thrombosis, etc. Eventually it would infect cancers and block their growth and metastasis. In addition, when this variant infects B-cells and T-cells, PAK18 boosts their immune system, and eventually produce antibodies against COVID and many other pathogenic viruses or bacteria [4]; (Figure 4). Thus, it might be called a “Trojan” horse carrying PAK18. Most importantly, PAK1-deficient mutant of both C. elegans and mice live 60% longer than the wild-type [10, 11].
Figure 4:PAK1-blockers: Stimulating “immune” system against viruses.
Concluding Remarks
In the case of RAF81, the minimum domain of RAF which binds RAS, A85K mutant in which Ala 85 of RAF is replaced by Lys, has 5 times higher affinity for RAS than the original RAF81. Thus, to optimize the efficiency of “Inspirin P”, we could try to select the “highest” affinity mutant of PAK18 for PIX by a PCR-mediated “random” mutation of PAK18. It has been known that R192A mutant of PAK18, in which Arg 192 is replaced by Ala, no longer blocks the PAK1-PIX interaction. Lastly, we must choose the most potent promoter of viral or cellular origin for the efficient production of PAK18.
A “potential” candidate for the potent translational promoter X in front of AUG codon is GGGGCC cluster (coding GlyAla cluster). This unique cluster in mRNA for a certain protein(s) has been known since 2013 to cause several neuronal diseases such as ALS, amyotrophic lateral sclerosis, mainly due to protein aggregates caused by GlyAla repeats [12]. This extra GGGGCC cluster promotes a so-called “RAN’ translation for “repeat associated non-AUG” translation, instead of a canonical AUG start codon, in association of phosphorylation of elongation factor (elf) 2alpha [13]. However, it is very unlikely that an extra GlyAla cluster attached to small peptides such as PAK18 could cause any “pathogenic” peptide aggregate.
References
- Coffey M, Strong JE, Forsyth PA, Lee PW (1998) Reovirus therapy of tumors with activated RAS pathway. Science 282(5392): 1332-1334.
- Bischoff J (1996) An adenovirus mutant that replicates selectively in p53-deficient human tumor cells. Science 274(5286): 373-376.
- Martuza R, Malick A, Markert JM, Ruffner KL, Coen DM (1991) Experimental therapy of human gliomas by means of a genetically engineered virus mutant. Science 252(5007): 854-856.
- Maruta H, He H (2020) PAK1-blockers: potential therapeutics against COVID-19. Med Drug Discov 6: 100039.
- Manser E, Leung T, Salihuddin H, Zhao ZS, Lim L (1994) A brain Ser/ Thr protein kinase activated by Cdc42 and Rac1. Nature 367: 40-46.
- Maruta H, Korn ED (1977) Acanthamoeba cofactor protein is a heavy chain kinase required for actin activation of the Mg2+-ATPase activity of Acanthamoeba myosin I. J Biol Chem 252(23): 8329-8332.
- Manser E, Loo TH, Koh CG, Zhao ZS, Chen XQ, et al. (1998) PAK kinases are directly coupled to the PIX family of nucleotide exchange factors. Mol Cell 1(2): 183-192.
- Obermeier A, Ahmed S, Manser E, Yen SC, Hall C, et al (1998) PAK promotes morphological changes by acting upstream of Rac. EMBO J 17(15): 4328-4339.
- He H, Hirokawa Y, Manser E, et al (2001) Signal therapy for RAS induced cancers in combination of AG 879 and PP1, specific inhibitors for ErbB2 and Src family kinases, that block PAK activation. Cancer J 7(3): 191-202.
- Yanase S, Luo Y, Maruta H (2013) PAK1-deficiency/down-regulation reduces brood size, activates HSP16.2 gene and extends lifespan in Caenorhabditis elegans. Drug Discov Ther 7(1): 29-35.
- Hawley E, Gehlhausen J, Karchugina S, Chow HY, Olivera DA, et al (2021) PAK1 inhibition reduces tumor size and extends the lifespan of mice in a genetically engineered mouse model of Neurofibromatosis Type 2 (NF2). Hum Mol Genet 30(17): 1607-1617.
- Goodman LD, Bonini NM (2019) Repeat-Associated Non-AUG (RAN) translation mechanisms are running into focus for GGGGCC-repeat associated ALS/FTD. Prog Neurobiol 183: 101697.
- Cheng W, Wang S, Mestre AA, Fu C, Makarem A, et al. (2018) C9ORF72 GGGGCC repeat-associated non-AUG translation is upregulated by stress through eIF2α phosphorylation. Nat Commun 9: 51.
© 2022 Hiroshi Maruta. This is an open access article distributed under the terms of the Creative Commons Attribution License , which permits unrestricted use, distribution, and build upon your work non-commercially.
 a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in
a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in 







.jpg)






























 Editorial Board Registrations
Editorial Board Registrations Submit your Article
Submit your Article Refer a Friend
Refer a Friend Advertise With Us
Advertise With Us
.jpg)






.jpg)














.bmp)
.jpg)
.png)
.jpg)










.jpg)






.png)

.png)



.png)






