- Submissions

Full Text
Modern Approaches in Drug Designing
Chemical Aspect of Antimicrobic Influence Mechanisms of Silver: One Possibility of Prevention from COVID-19
Azra Jaganjac1*, Lejla Klepo1 and Dragan Kresic2
1Department of Chemistry, Bosnia and Herzegovina
2Largest industrial drug manufacturer in B & H, Bosnia and Herzegovina
*Corresponding author: Azra Jaganjac, Department of Chemistry, Bosnia and Herzegovina
Submission: June 15, 2020;Published: October 08, 2021

ISSN: 2576-9170 Volume3 Issue4
Introduction
In December 2019, a pneumonia outbreak was reported in Wuhan, China. On 31 December 2019, the outbreak was traced to a novel strain of coronavirus, which was given the interim name 2019-nCoV by the World Health Organization (WHO), later renamed SARS-CoV-2 by the International Committee on Taxonomy of Viruses. The pandemic has resulted in travel restrictions and nationwide lockdowns in many countries. Since the main way to prevent the spread of this disease around the world is disinfection and hygiene, it seems to us that the use of the bactericidal effect of silver shown in this paper would be interesting for further fight against this plague. Silver can be found in native form, alloyed with gold or combined with sulfur, arsenic, antimony or chlorine in ores such as argentite (Ag2S), horn silver (AgCl), and pyrargyrite (Ag3SbS3). Pure silver has the highest thermal conductivity and electrical conductivity. Silver is used in photography, electronics, for making mirrors and optics, in solder and brazing, as money and as catalyst.
History of Using Silver as a Medicine
The word “silver” appears in Anglo-Saxon in various spellings such as seolfor and siolfor. A similar form is seen throughout the Teutonic languages (compare Old High German silabar and silbir). The symbol “Ag” is from the Latin for “silver”, argentum (compare Greek αργυρος (argyros)), from the Indo-European root argmeaning “white” or “shining”. The oldest object made form silver was found in Egypt in 4157 B.C. Hippocrates, the father of modern medicine, wrote that silver had beneficial healing and anti-disease properties, and the Phoenicians used to store water, wine, and vinegar in silver bottles to prevent spoiling. Monks in the monasteries used silver pots to keep the water before putting it in baptismal font, and in orthodox churches all icons are put in silver frames, and in Mecca, and the black stone in the wall of the Kaaba is encrusted with silver, and no one has ever been infected by touching and kissing them. In Middle Ages royalty used the silver pots, plates, knifes, spoons, forks etc, and they did not suffer from the plague. Silver compounds were used successfully to prevent infection in World War I before the advent of antibiotics. Silver nitrate solution was a standard of care but was largely replaced by silver sulfadiazine cream (SSD Cream) which was generally the “standard of care” for the antibacterial and antibiotic treatment of serious burns until the late 1990s [1].
Experimental
Silver ions and silver compounds show a toxic effect on some bacteria, viruses, algae and fungi, typical for heavy metals like lead or mercury, but without the high toxicity to humans that is normally associated with them. Its germicidal effects kill many microbial organisms in vitro. The anti-microbial properties of silver stem from the chemical properties of its ionized form, Ag+ (Figure 1). This ion forms strong molecular bonds with other substances used by bacteria to respire, such as molecules containing sulfur, nitrogen, and oxygen. Once the Ag+ ion complexes with these molecules, they are rendered unusable by the bacteria, depriving it of necessary compounds and eventually leading to the bacteria’s death [2]. Also today, various kinds of silver compounds, or devices to make solutions or colloids containing silver, are sold as remedies for a wide variety of diseases. Not only that colloidal silver and ion silver have antimicrobial effect on some bacteria, bat some silver complexes have the same effect, like [Ag(imd)] n (Himd = imidazole, C3H4N2), diphosphine complexes [Ag(P-P)2]NO3, which showed wide spectra in effective antimicrobial activities against bacteria [3,4].
Structure of Prokaryotic cell Prokaryotes are single-celled organisms that lack a nucleus and that do not have their genetic material organized into chromosomes. They constitute the Kingdom Monera, which is split into two divisions: the Schizophyta (traditionally called bacteria) and the Cyanophyta (formerly known as blue-green algae). On Figure 2 are some examples of prokaryotic cells. Chemical compounds of bacteria the most important molecule in the cell are water. Whole life depends on its special properties. Water makes up over 70 % of all living organisms by weight. Most of compounds of living beings are in solution inside cells. Hydrogen bonding plays a very important role in giving water the properties which are required for life. The polar nature of water also accounts for its ability to dissolve a large number of compounds. Water has been referred to as the universal solvent of life because it dissolves so many compounds which must contain atoms with positive or negative charges. When placed in water, they ionize or split in their component charged atoms. Water containing dissolved substances freezes at the lower temperature than pure water. Because the water molecules are hydrogen bonded to the dissolved ions, a much lower temperature is required for the water molecules to assume the rigid lattice structure of ice. Another important property of every aqueous solution is its degree of acidity. Bacteria often produce acids and less commonly, bases when they degrade compounds to gain energy. All cells contain a variety of small organic and inorganic molecules, many which occur in the form of ions. About 1% of the weight of bacterial cell, once the water is removed (dry weight), is composed of inorganic ions, principally Na+ (sodium), K+ (potassium), Mg2+ (magnesium), Ca2+ (calcium), Fe2+ (iron), Cl- (chloride), PO4 3- (phosphate) and SO4 2- (sulfate) (Figure 3).
Positively charged ions are required in minute amounts in order for certain enzymes to function. The negatively charged phosphate ion play a key role in energy metabolism. The small molecules include compounds that have accumulated in the process of metabolism of sugars to supply the cell with energy. These are precursor metabolites which are converted to building blocks of large molecules, the macromolecules. The most important small organic molecule is Adenosine Triphosphate (ATP), the storage from of energy in the cell. It is composed of the sugar ribose, the purine, adenine, and three phosphate groups arranged in tandems. The breakage of terminal high-energy bond of ATP results in formation of Adenosine Diphosphate (ADP), and the release of energy. Macromolecules are building material of cells. The four major classes of macromolecules are proteins, polysaccharides, nucleic acids and lipid. These four groups of macromolecules differ each other in their chemical structure [5,6]; (Figure 4).
Figure 1: Some items that were used in Middle age.

Figure 2: Prokaryotic cells.
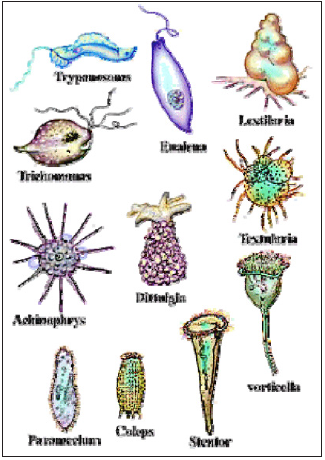
Figure 3: Bacteria cell.
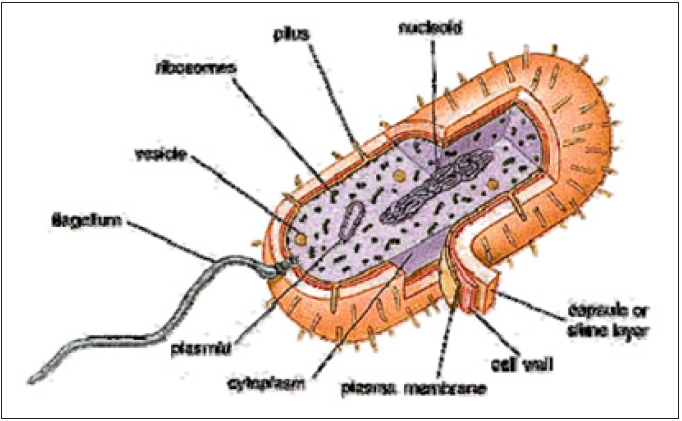
Figure 4: Some items that were used in Middle age.

Bacteria Cell
The cell is considered to be the smallest structure in biology that has all the properties of living things and an understanding of cells, and the basics of cell structure and function is critical to making sense out of biology. Bacteria, despite their apparent simplicity, contain a well-developed cell structure which is responsible for many of their unique biological properties. This bacterial cell is consisting from: nucleotide (a nuclear region where the chromosomal matter is found), plasmids (prokaryotes often have small loops of DNA called plasmids which can be transferred to other cells), ribosome (give the cytoplasm of bacteria a granular appearance in electron micrographs), vesicles (prokaryotic cells contain a number of vesicles and they store, transport, or digest cellular product and wastes), pilus (hair like structures made of protein allow bacteria to attach to other cells), flagellum, cytoplasm, and the surface of the cell include capsule (a layer of polysaccharide (sometimes proteins) that protects the bacterial cell and is often associated with pathogenic bacteria because it serves as a barrier against phagocytosis by white blood cells), cell wall, and plasma membrane.
Plasma Membrane
The cell membrane is a fluid mosaic of lipids, proteins, and carbohydrates. Lipids consist mostly of hydrocarbons. Smaller than true (polymeric) macromolecules, lipids are a highly varied group in both form and function and include such things as waxes and certain pigments. The most important are three classes of lipids: the fats, steroids (animal), and phospholipids. A fat is constructed from two kinds of smaller molecules: glycerol and fatty acids. In making a fat, three fatty acids each join to glycerol by an ester linkage, a bond between a hydroxyl group and a carboxyl group. The resulting fat, also called a triacylglycerol or triglyceride, thus consists of three fatty acids linked to one glycerol molecule. The major function of fats is energy storage. Phospholipids are major components of the cell membrane. They are similar to fats but have only two fatty acids rather than three. The phospholipid bilayer forms a semi-permeable boundary between the cell and its external environment. Proteins are the most structurally sophisticated molecules known, and account for more than 50% of the dry weight of most cells. They are all polymers constructed from the same set of 20 amino acids (Figure 5).
Figure 5: Black Stone at Kaaba.

Membrane proteins are classified into two major categories, Integral proteins and Peripheral proteins. Integral proteins are generally transmembrane proteins, with hydrophobic regions that completely span the hydrophobic interior of the membrane. Peripheral proteins are not embedded in the lipid bilayer at all; they are loosely bound to the surface of the membrane, often to the exposed parts of integral proteins. Membrane carbohydrates are usually branched oligosaccharides with fewer than 15 sugar units. Some of these oligosaccharides are covalently bonded to lipids, forming molecules called glycolipids. Most are covalently bonded to proteins, which are thereby glycoproteins.
Cell Wall
Bacteria cells have a rigid cell wall. Cell walls of bacteria contain polysaccharides as major components but not chitin or cellulose. Two of the chief sugars are glucosamine and a derivative of galactosamine called muramic acid. Lipids and amino acids are also found in the cell [7].
Result and Discussion
Silver as a good bactericide has been known since ancient times. It was used to store water, wine, holly water and vinegar in silver bottles to prevent spoiling. In middle-aged royalty used silver for making glasses, pots, and plates, knifes, spoons, forks etc., and because of this no one of the royal family members weren’t infected with plague. Even today, silver is used in religious facilities to prevent hand and mouth infections. It was obvious that silver was responsible for this effect. Because silver ions and colloidal silver have antimicrobial effect in this work, we will try to see which silver is more efficient and try to explain on which mechanism these reactions are happening. What we want is to show how we can use silver in medicine, how it effects on bacteria. It may even help prevent the spread of the coronavirus around the globe.
References
- Chang TW, Weinstein L (1975) Prevention of herpes keratoconjunctivitis in rabbits by silver sulfadiazine. Antimicrob Agents Chemother 8(6): 677-678.
- Slawson RM, Van Dyke MI, Lee H, Trevors JT (1992) Germanium and silver resistance, accumulation and toxicity in microorganisms. Plasmid 27(1): 72-79.
- Cuin A, Massabni A, Leite C, Daisy NS, Neves A, et al. (2006) Synthesis, X-ray structure and antimycobacterial activity of silver complexes with -hydroxycarboxylic acids. J Inorg Biochem 101(2): 291-296.
- Nomiya K, Tsuda K, Sudoh T, Oda M (1997) Ag(I)-N bond-containing compound showing wide spectra in effective antimicrobial activities: Polymeric silver (I) imidazolate. J Inorg Biochem 68(1): 39-44.
- Galbraith DI, Wilson GD (1966) Biological Science. Holt, Rinehart and Winston of Canada, Canada.
- Nester WE, Anderson DG, Roberts CE, Pearsall NN, Nester MT (2004) Microbiology: A human perspective. University of Washington, University of Georgia, USA.
- Demchick PH, Koch AL (1996) The permeability of the wall fabric of Escherichia coli and Bacillus subtilize. Journal of Bacteriology 178 (3): 768-773.
© 2021 Azra Jaganjac. This is an open access article distributed under the terms of the Creative Commons Attribution License , which permits unrestricted use, distribution, and build upon your work non-commercially.
 a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in
a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in 







.jpg)






























 Editorial Board Registrations
Editorial Board Registrations Submit your Article
Submit your Article Refer a Friend
Refer a Friend Advertise With Us
Advertise With Us
.jpg)






.jpg)














.bmp)
.jpg)
.png)
.jpg)










.jpg)






.png)

.png)



.png)






