- Submissions

Full Text
Modern Approaches in Drug Designing
LC-MS and GC-MS-Based Assessment of the Impact of Consciousness Energy Healing Treatment on the Isotopic Abundance Ratios of Sulfamethoxazole
Mahendra Kumar Trivedi1, Alice Branton1, Dahryn Trivedi1 and Snehasis Jana2*
1Trivedi Global, Inc., Henderson, USA
2Trivedi Science Research Laboratory Pvt. Ltd., India
*Corresponding author: Jana S, Trivedi Science Research Laboratory Pvt. Ltd., Thane (W), Maharashtra, India
Submission: May 26, 2021;Published: June 18, 2021

ISSN: 2576-9170 Volume3 Issue3
Abstract
Sulfamethoxazole is an antibiotic used for the treatment of infections caused by bacteria. The experiment was performed to evaluate the impact of the Trivedi Effect® on the structural properties and the isotopic abundance ratio of sulfamethoxazole using LC-MS and GC-MS analytical techniques. Sulfamethoxazole sample was divided into two parts, one part of sulfamethoxazole was considered as a control sample, while the other part only received the Consciousness Energy Healing Treatment remotely by a wellknown Spiritual Energy Healer, Mr. Mahendra Kumar Trivedi and termed as a treated sample. In both the samples, LC-MS spectra showed at retention time (Rt) 2.51 minutes, that exposed the mass of the deprotonated molecular ion peak at m/z 252 [M-H]- (calculated for C10H10N3O3S-, 252.04). The peak area of the treated sulfamethoxazole was significantly increased by 55.17% than untreated test item. The LC-MS-based isotopic abundance ratio of PM+1/PM in the Biofield Treated/Blessed sulfamethoxazole was significantly decreased by 55.57% than untreated. Similarly, the GC-MS peak area% of the treated sulfamethoxazole was significantly increased by 12.96% than untreated test item. The GC-MS-based isotopic abundance ratio of PM+1/PM and PM+2/PM in the Biofield Treated/Blessed sulfamethoxazole was significantly decreased by 15.86% and 8.8%, respectively than untreated test item. The isotopic abundance ratios of PM+1/PM (2H/1H or 13C/12C or 15N/14N or 17O/16O or 33S/32S) and PM+2/PM (18O/16O or 34S/32S) in the treated sulfamethoxazole were significantly reduced than untreated test item. Thus, 13C, 2H, 15N, 17O, 18O, 33S, and 34S contributions from (C10H11N3O3S)+ to m/z 254 and 255 in the treated sample were significantly reduced than untreated test item. The reduced isotopic abundance ratios would highly influence the atomic bond vibration, chemical bond strength, and the stability of treated sulfamethoxazole. It can be envisaged that the changes in peak area%, isotopic abundance, and mass peak intensities could be due to changes in nuclei, possibly through the interference of neutrino particles via the Trivedi Effect®. The new form of sulfamethoxazole would be more efficacious pharmaceutical formulations that might offer better solubility, dissolution, absorption, bioavailability, and better therapeutic response against urinary tract infections, tuberculosis, diarrhoea, ear infections, bronchitis, shigellosis, and Pneumocystis jiroveci pneumonia, etc.
Keywords: Sulfamethoxazole; Biofield energy; The Trivedi effect®; Consciousness energy healing treatment; Isotopic abundance ratio
Introduction
Sulfamethoxazole is an antibiotic that is very commonly used in the treatment of infections
caused by bacteria. Sulfamethoxazole act by inhibit bacterial nucleotides and DNA and kill the
bacteria by inhibiting the bacterial synthesis of dihydro folic acid competitively [1,2]. It is
used for the therapeutic management of urinary tract infections, ear infections, bronchitis,
tuberculosis, shigellosis, traveller’s diarrhoea, and Pneumocystis jiroveci pneumonia
[3]. The side effects associated with sulfamethoxazole therapy are nausea, vomiting, loss
of appetite, and skin rashes. It rapidly absorbed orally as well as topically. The stability of
any pharmaceutical compound depends upon its physicochemical properties and adds an important role in its dissolution, absorption, and bioavailability
to achieve a better therapeutic value [4-7]. The Biofield Energy
Healing Treatment simultaneously proved to have a significant
impact on the particle size, surface area, thermal behaviour, and
bioavailability of the pharmaceutical/nutraceutical compounds
[8-10]. The Trivedi Effect® - a natural and scientifically established
phenomenon in which an individual expert can harness an inherent
intelligent energy from the Universe and transfer it anywhere on
the planet via the probable form of neutrinos [11]. Biofield Energy
is an “electromagnetic energy field” which present surrounding
the living systems, produced by the constant movement of
the electrically charged particles (cells, ions, etc.) inside the
body [12,13]. Biofield Energy-based therapies have significant
outcomes against various diseases [14]. The National Center of
Complementary and Integrative Health (NCCIH) has recognized and
accepted “Biofield Energy Healing Therapy” as a Complementary
and Alternative Medicine (CAM) in health care approach along with
the other therapies, medicines, and practices, i.e., Ayurveda, Chinese
herb and medicine, Tai Chi, yoga, Qi Gong, Reiki, hypnotherapy, etc.
[15]. These CAM therapies have been widely utilized by most of the
American population with advantages [16]. Mr. Trivedi’s Blessing
has the outstanding capability to alter the characteristic properties
of the several non-living materials and living object(s), i.e., ceramic,
metals, and organic compounds, microbes, crops, cancer cells [17-
26], etc. The Consciousness Energy Healing Treatment has also
altered the isotopic abundance ratio of the pharmaceutical and
nutraceutical compounds [27,28].
Analysis of stable isotopes possess wide spectrum uses
in various scientific fields for perception the “isotope effects”
resulting from the alteration of “isotopic composition” of a
molecule [29,30]. The isotope ratio analysis can be done using Mass
Spectrometry (MS) techniques such As Liquid Chromatography
- Mass Spectrometry (LC-MS) and Gas Chromatography - Mass
Spectrometry (GC-MS) in low micromolar concentration with
sufficient precision [30,31]. The Biofield Energy Healing/Blessing
Treatment could be an economical approach for designing better
pharmaceutical formulations. Thus, the LC-MS and GC-MS were
used in this experiment to characterize the structural properties
and assess the isotopic abundance ratio of PM+1/PM and PM+2/PM in
the Biofield Treated/Blessed sulfamethoxazole as compared to the
control sample.
Materials and Methods
Chemicals and reagents
The sulfamethoxazole powder test sample was purchased from Sigma Aldrich, USA, and other chemicals and solvents like acetonitrile, methanol, and formic acid were of analytical grade purchased from Merck, India.
Consciousness energy healing treatment strategies
The sulfamethoxazole powder sample was divided into two equal parts and termed as untreated and treated. The untreated sample did not receive the Biofield Energy Treatment/Blessing; while the treated with a “sham” healer a person who did not aware about Biofield Energy or Blessing. However, the Biofield Treated/ Blessed sulfamethoxazole was received the Biofield Energy Healing/Blessing Treatment remotely for ~3 minutes by Mr. Mahendra Kumar Trivedi, USA, a renowned Spiritual Energy Healer. After Blessing, both the untreated and Biofield Treated samples were kept in sealed conditions and characterized using LC-MS and GC-MS, analytical techniques.
Characterization
Liquid Chromatography-Mass Spectrometry (LC-MS) analysis and calculation of isotopic abundance ratio: The LC-MS analysis of the sulfamethoxazole was carried out with the help of LC-MS ThermoFisher Scientific (USA), equipped with an ion trap detector connected with a triple-stage quadrupole mass spectrometer. A reversed phase Thermo Scientific Synchronis C18 (Length-250mm X ID 4.6mm X 5micron) column was used and maintained at 25 °C. Methanol was the diluent used for the sample preparation. 5μL of sulfamethoxazole solution was injected, and the analyte was eluted using acetonitrile + 0.1% formic acid (75:25) pumped at a constant flow rate of 0.5mL/min. Chromatographic separation was achieved using gradient condition and the total run time was 10min. Peaks were monitored at 254nm using the PDA detector. The mass spectrometric analysis was performed in -ve ESI mode. The natural abundance of each isotope (C, H, N, O, and S) can be predicted from the comparison of the height of the isotope peak with respect to the base peak. The values of the natural isotopic abundance of the common elements are obtained from the literature [30,32-34]. The LC-MS-based isotopic abundance ratios (PM+1/PM) for the control and Biofield Energy Treated sulfamethoxazole were calculated using equation 1.
% Change in isotopic abundance ratio = [(IARTreated - IARControl)/ IARControl) x 100] (1)
Where IARTreated = isotopic abundance ratio in the treated sulfamethoxazole and IARControl = isotopic abundance ratio in the control sulfamethoxazole.
Gas Chromatography-Mass Spectrometry (GC-MS) analysis: GC-MS of the sulfamethoxazole was analyzed with the help of Perkin Elmer Gas chromatograph equipped with a PE-5MS (30M x 250micros x 0.250microns) capillary column and coupled to a single quadrupole mass detector was operated with Electron Impact (EI) ionization in positive mode. The oven temperature was programmed from 75 °C (5 min hold) to 280 °C (14.5 min hold) @ 10°C /min (total run time 40min). The sample was prepared taking 60mg of the sulfamethoxazole in 4ml acetonitrile and water (1:1) as a diluent. The GC-MS based isotopic abundance ratios (PM+1/PM and PM+2/PM for the control and Biofield Energy Treated sulfamethoxazole was calculated using equation 1.
Results and Discussion
Liquid Chromatography-Mass Spectrometry (LC-MS)
The chromatogram of both the sulfamethoxazole samples shown in Figure 1. The chromatograms showed the single major chromatographic peak at the retention time (Rt) of 2.51 minutes (Figure 1). But, the peak area of the Biofield Energy Treated sulfamethoxazole was significantly increased by 55.17% compared to the control sample, which indicated that the solubility profile of the Biofield Energy Treated sulfamethoxazole was significantly increased compared to the control sample. The sulfamethoxazole was detected with the molecular mass peak [M-H]- at m/z 252 in the MS spectrum in negative ion mode [35]. The mass spectra of both the samples of sulfamethoxazole (Figure 2) exhibited the mass of the deprotonated molecular ion peak at m/z 252 [M-H]- (calculated for C10H10N3O3S-, 252.04).
Figure 1: Liquid chromatograms of the control and biofield energy treated sulfamethoxazole.
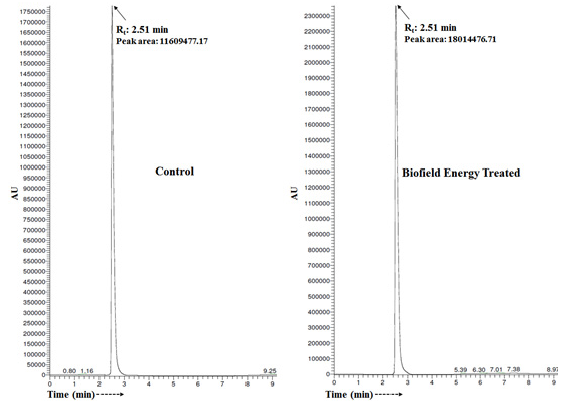
Figure 2: Mass spectra of the control and biofield energy treated sulfamethoxazole at Rt 2.5 minutes.
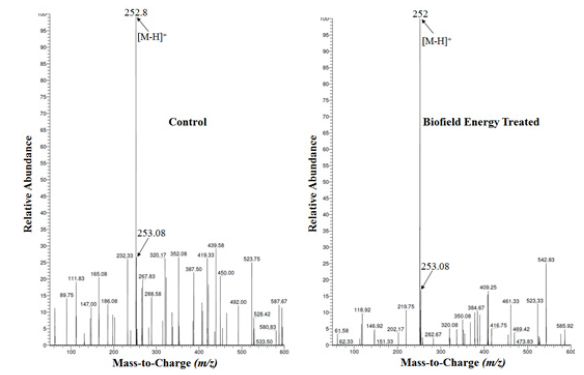
The LC-MS spectra of both the samples showed the mass of the
molecular ion peak at m/z 252 [M-H]- (calculated for C10H10N3O3S-,
252.04) with relative intensity of 100%. The theoretical calculation
of PM+1 for sulfamethoxazole was presented as below:
P (13C) = [(10 x 1.1%) x 100% (the actual size of the M- peak)]
/ 100% = 11%
P (2H) = [(10 x 0.015%) x 100%] / 100%= 0.15%
P (15N) = [(3 x 0.4%) x 100%] / 100% = 1.2%
P (17O) = [(3 x 0.04%) x 100%] / 100% = 0.12%
P (33S) = [(1 x 0.75%) x 100%] / 100% = 0.75%
PM+1, i.e. 13C, 2H, 15N, 17O and 33S contributions from (C10H10N3O3S)-
to m/z 253 = 13.22%
Based on the above calculation, it has been observed that 13C,
15N, and 33S have major contribution to m/z 253.
The LC-MS-based isotopic abundance ratio analysis PM and
PM+1 for sulfamethoxazole near m/z 252 [M+] and 253 [(M+1)+],
respectively of the control and Biofield Energy Treated samples in
the ESI-MS spectra (Table 1). The isotopic abundance ratio (PM+1/PM)
in the Biofield Energy Treated sulfamethoxazole was significantly
decreased by 55.57% compared with the control sample (Table 1).
Thus, it was concluded that the 13C, 2H, 15N, 17O, and 33S contributions
from (C10H10N3O3S)- to m/z 253 in the treated sample were
significantly decreased compared to the control sample.
Table 1: LC-MS based isotopic abundance analysis results in Biofield Energy Treated sulfamethoxazole compared to the control sample.
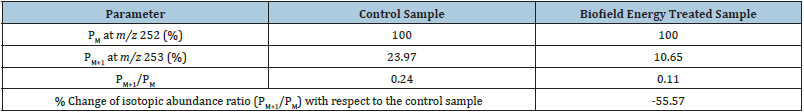
PM: the relative peak intensity of the parent molecular ion [M+]; PM+1: the relative peak intensity of the isotopic molecular ion [(M+1)+], M: mass of the parent molecule.
Gas Chromatography-Mass Spectrometry (GC-MS) analysis
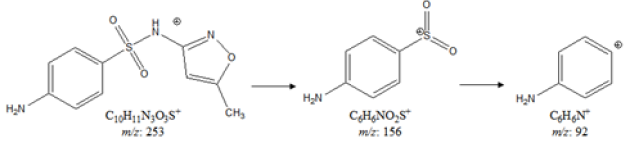
The control and Biofield Energy Treated/Blessed sulfamethoxazole showed the presence of a sharp chromatographic peak at the retention time of 17.25 and 17.49 minutes, respectively in the gas chromatograms (Figure 3 & Figure 4). The peak area% of the Biofield Treated sample was significantly increased by 12.96% as compared to the untreated sulfamethoxazole. This indicated that the solubility of the treated sulfamethoxazole was significantly increased compared to the control sample. The peak near the Rt of 17 min in both the chromatograms indicating the sulphanilamide present in the sample. The parent molecular ion peak of sulfamethoxazole at m/z 253 [M]+ (calculated for C10H11N3O3S+, 253.05) in both the samples, along with the fragment ion peaks near m/z 156 and 92 (Figure 3 & Figure 4) corresponded to the molecular formula C6H6NO2S+ and C6H6N+, respectively (Figure 5).
Figure 3: The GC-MS chromatogram and mass spectra of the control sulfamethoxazole.
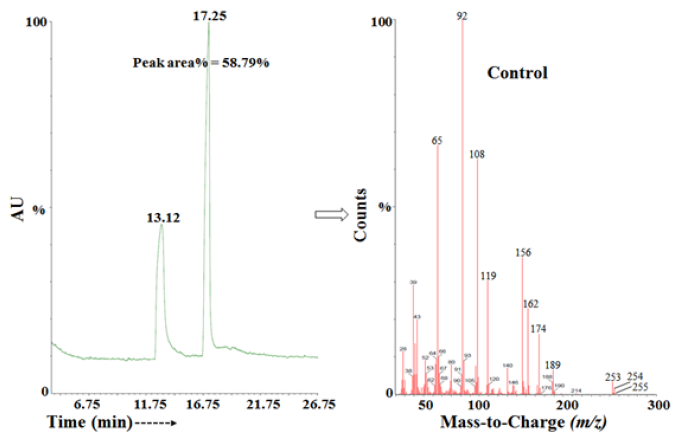
Figure 4: The GC-MS chromatogram and mass spectra of the biofield energy treated sulfamethoxazole.
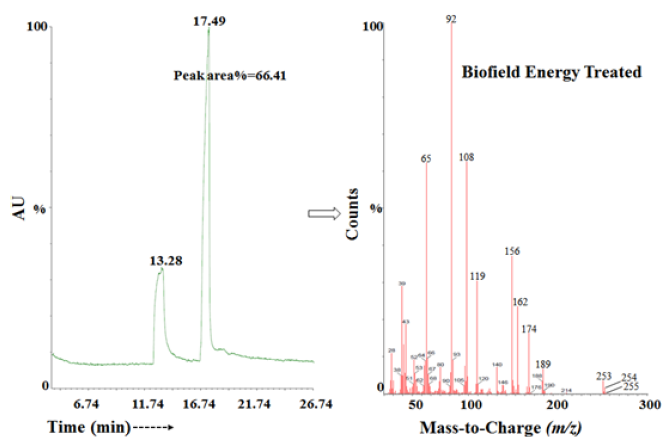
Figure 5: Proposed fragmentation pattern of sulfamethoxazole.
The GC-MS spectra of both the control and treated
sulfamethoxazole showed the mass of the molecular ion peak
[M]+ at m/z 253 [M]+ (calculated for C10H11N3O3S+, 253.05). As per
theory-based calculation of PM+1 and PM+2 for sulfamethoxazole was
presented as below:
P (13C) = [(10 x 1.1%) x 3.37% (the actual size of the M+ peak)]
/ 100% = 0.37%
P (2H) = [(11 x 0.015%) x 3.37%] / 100%= 0.005%
P (15N) = [(3 x 0.4%) x 3.37%] / 100% = 0.04%
P (17O) = [(3 x 0.04%) x 3.37%] / 100% = 0.004%
P (33S) = [(1 x 0.75%) x 3.37%] / 100% = 0.025%
PM+1, i.e. 13C, 2H, 15N, 17O, and 33S contributions from (C10H11N3O3S)+
to m/z 254 = 0.44%
Similarly,
P (18O) = [(3 x 0.2%) x 3.37%] / 100% = 0.002%
P (34S) = [(1 x 4.21%) x 3.37%] / 100% = 0.14%
PM+2, i.e., 34S and 18O contributions from (C10H11N3O3S)+ to m/z
255 = 0.14%
Based on the above calculation, it has been observed that 13C,
15N, 33S, and 34S have major contribution to m/z 254 and 255.
The GC-MS based isotopic abundance ratio analysis of
the Biofield Energy Treated sulfamethoxazole samples was
calculated compared to the control sample. PM, PM+1, and PM+2
for sulfamethoxazole near m/z 253 [M+], 254 [(M+1)+], and 255
[(M+2)+] were obtained from the observed relative peak intensities
from the mass spectra (Table 2). The isotopic abundance ratio of
PM+1/PM and PM+2/PM in the Biofield Treated sulfamethoxazole was
significantly decreased by 15.86% and 8.8%, respectively compared
with the control sample (Table 2). Hence, 13C, 2H, 15N, 17O, 18O, 33S,
and 34S contributions from (C10H11N3O3S)+ to m/z 254 and 255 in
the Biofield Energy Treated sample were significantly decreased
compared to the control sample.
Table 2: GC-MS based isotopic abundance analysis results of Biofield Energy Treated sulfamethoxazole compared to the control samples.
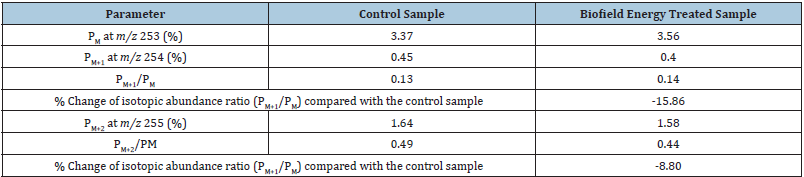
PM: the relative peak intensity of the parent molecular ion [M+]; PM+1: the relative peak intensity of the isotopic molecular ion [(M+1)+]; PM+2: the relative peak intensity of the isotopic molecular ion [(M+2)+]; M: mass of the parent molecule.
LC-MS and GC-MS characterizations confirmed the structure of the sample as sulfamethoxazole. The isotopic abundance ratios of PM+1/PM (2H/1H or 13C/12C or 15N/14N or 17O/16O or 33S/32S) and PM+2/PM (18O/16O or 34S/32S) in the Biofield Energy Treated sulfamethoxazole were significantly decreased compared to the control sample. The isotopic composition alteration in the Biofield Treated/Blessed sulfamethoxazole might be due to alteration in nuclei through neutrinos via Trivedi’s Blessing. The neutrinos have the ability to interact with protons and neutrons in the nucleus, which indicated a close relation between neutrino and the isotope formation [11,30,31]. The reduced isotopic abundance ratios would highly influence the atomic bond vibration, chemical bond strength, and the stability of treated sulfamethoxazole [36,37]. The Consciousness Energy Healing Treatment might create a new form of sulfamethoxazole which would show better solubility, dissolution, absorption, and bioavailability than the untreated test sample. The Consciousness Energy Healing Treated sulfamethoxazole would be more efficacious for the prevention and treatment of urinary tract infections, ear infections, shigellosis, traveler’s diarrhoea, bronchitis, and Pneumocystis jiroveci pneumonia, etc.
Conclusion
The outcomes of this experiment showed a significant impact on the peak area%, isotopic abundance ratios and mass peak intensities of sulfamethoxazole. The liquid chromatography peak area of the Biofield Energy Treated sulfamethoxazole was significantly increased by 55.17% with respect to untreated. The LC-MS based isotopic abundance ratio of PM+1/PM in the Biofield Treated/Blessed sulfamethoxazole was significantly decreased by 55.57% with respect to untreated. Similarly, the GC-MS peak area% of the Biofield Energy Treated sulfamethoxazole was significantly increased by 12.96% compared to the control sample. The GC-MS-based isotopic abundance ratio of PM+1/PM and PM+2/ PM was significantly decreased by 15.86% and 8.8%, respectively in the Biofield Treated/Blessed sulfamethoxazole than untreated. The isotopic abundance ratios of PM+1/PM (2H/1H or 13C/12C or 15N/14N or 17O/16O or 33S/32S) and PM+2/PM (18O/16O or 34S/32S) in the Biofield Energy Treated sulfamethoxazole were significantly reduced compared to the control sample. Thus, 13C, 2H, 15N, 17O, 18O, 33S, and 34S contributions from (C10H11N3O3S)+ to m/z 254 and 255 in the Biofield Energy Treated sample were significantly reduced compared with the control sample. The reduced isotopic abundance ratios would highly influence the atomic bond vibration, chemical bond strength, and the stability of Biofield Energy Treated sulfamethoxazole. The changes in isotopic abundance, mass peak intensities, and peak area% could be due to alteration of nuclei possibly through the interference of neutrino particles via the Trivedi Effect®. The new form of sulfamethoxazole would be more efficacious pharmaceutical formulations that might offer better solubility, dissolution, absorption, bioavailability, and better therapeutic response against urinary tract infections, tuberculosis, diarrhoea, ear infections, bronchitis, shigellosis, and Pneumocystis jiroveci pneumonia, etc.
Acknowledgement
The authors are grateful to Sophisticated Instrumentation Centre for Applied Research & Testing (SICART) India, Trivedi Science, Trivedi Global, Inc., and Trivedi Master Wellness for their assistance and support during this work.
References
- Zander J, Besier S, Ackermann H, Wichelhaus TA (2010) Synergistic antimicrobial activities of folic acid antagonists and nucleoside analogs. Antimicrob Agents Chemother 54(3): 1226-1231.
- Neu HC, Gootz TD (1996) Antimicrobial chemotherapy. In: Baron S (Ed.), Medical Microbiology. (4th edn), University of Texas Medical Branch at Galveston, Galveston TX, USA.
- Brunton L, Chabner BA, Knollman B (2011) Goodman and Gilman’s The pharmacological Basis of Therapeautics. (12th edn), The McGraw-Hill Companies, Inc, New York, USA.
- Close SJ, McBurney CR, Garvin CG, Chen DC, Martin SJ (2002) Trimethoprim-sulfamethoxazole activity and pharmacodynamics against glycopeptide-intermediate Staphylococcus aureus. Pharmacotherapy 22(8): 983-989.
- Mulla SI, Hu A, Sun Q, Li J, Suanon F, Ashfaq M, Yu CP (2018) Biodegradation of sulfamethoxazole in bacteria from three different origins. J Environ Manage 206: 93-102.
- Savjani KT, Gajjar AK, Savjani JK (2012) Drug solubility: Importance and enhancement techniques. ISRN Pharmaceutics, 195727.
- Khadka P, Ro J, Kim H, Kim I, Kim JT, et al. (2014) Pharmaceutical particle technologies: An approach to improve drug solubility, dissolution and bioavailability. Asian J Pharm 9(6): 304-316.
- Trivedi D, Trivedi MK, Branton A, Nayak G, Jana S (2019) Consciousness energy healing treatment: Impact on the physicochemical and thermal properties of ascorbic acid. Food Nutr Current Res 2(2): 164-173.
- Nayak G, Trivedi MK, Branton A, Trivedi D, Jana S (2018) Consciousness energy healing treatment: Impact on physicochemical and thermal properties of silver sulfadiazine. Journal of Advanced Pharmaceutical Science and Technology 2(1): 1-13.
- Branton A, Jana S (2017) The influence of energy of consciousness healing treatment on low bioavailable resveratrol in male sprague dawley rats. International Journal of Clinical and Developmental Anatomy 3(3): 9-15.
- Trivedi MK, Mohan TRR (2016) Biofield energy signals, energy transmission and neutrinos. American Journal of Modern Physics 5(6): 172-176.
- Rubik B (2002) The biofield hypothesis: Its biophysical basis and role in medicine. J Altern Complement Med 8(6): 703-717.
- Nemeth L (2008) Energy and biofield therapies in practice. Beginnings 28(3): 4-5.
- Rubik B, Muehsam D, Hammerschlag R, Jain S (2015) Biofield science and healing: History, terminology, and concepts. Glob Adv Health Med 4: 8-14
- Koithan M (2009) Introducing complementary and alternative therapies. J Nurse Pract 5(1): 18-20.
- Barnes PM, Bloom B, Nahin RL (2008) Complementary and alternative medicine use among adults and children: United States, 2007. Natl Health Stat Report 12: 1-23.
- Trivedi MK, Branton A, Trivedi D, Nayak G, Panda P, et al. (2016) Gas chromatography-mass spectrometric analysis of isotopic abundance of 13C, 2H, and 18O in biofield energy treated p-tertiary butylphenol (PTBP). American Journal of Chemical Engineering 4(4): 78-86.
- Trivedi MK, Branton A, Trivedi D, Nayak G, Sethi KK, et al. (2016) Gas chromatography-mass spectrometry based isotopic abundance ratio analysis of biofield energy treated methyl-2-napthylether (Nerolin). American Journal of Physical Chemistry 5(4): 80-86.
- Trivedi MK, Patil S, Tallapragada RM (2013) Effect of biofield treatment on the physical and thermal characteristics of vanadium pentoxide powders. J Material Sci Eng S 11: 001.
- Trivedi D, Trivedi MK, Branton A, Nayak G, Jana S (2019) Consciousness energy healing treatment: Impact on physicochemical and thermal properties of zinc. Modern Approaches on Material Science. Mod App Matrl Sci 1(4): 95-101.
- Trivedi MK, Branton A, Trivedi D, Nayak G, Mondal SC, et al. (2015) Antimicrobial sensitivity, biochemical characteristics and biotyping of Staphylococcus saprophyticus: An impact of biofield energy treatment. J Women’s Health Care 4: 271.
- Trivedi MK, Branton A, Trivedi D, Nayak G, Shettigar H, et al. (2015) Antibiogram of multidrug-resistant isolates of Pseudomonas aeruginosa after biofield treatment. J Infect Dis Ther 3: 244.
- Trivedi MK, Branton A, Trivedi D, Nayak G, Mondal SC, et al. (2015) Evaluation of plant growth regulator, immunity and DNA fingerprinting of biofield energy treated mustard seeds (Brassica juncea). Agriculture, Forestry and Fisheries 4(6): 269-274.
- Trivedi MK, Branton A, Trivedi D, Nayak G, Bairwa K, et al. (2015) Physical, thermal, and spectroscopic characterization of biofield energy treated murashige and skoog plant cell culture media. Cell Biology 3(4): 50-57.
- Trivedi MK, Patil S, Shettigar H, Mondal SC, Jana S (2015) The potential impact of biofield treatment on human brain tumor cells: A time-lapse video microscopy. J Integr Oncol 4: 141.
- Trivedi MK, Patil S, Shettigar H, Gangwar M, Jana S (2015) In vitro evaluation of biofield treatment on cancer biomarkers involved in endometrial and prostate cancer cell lines. J Cancer Sci Ther 7: 253-257.
- Trivedi MK, Branton A, Trivedi D, Nayak G, Sethi KK, et al. (2016) Isotopic abundance ratio analysis of biofield energy treated indole using gas chromatography-mass spectrometry. Science Journal of Chemistry 4(4): 41-48.
- Trivedi MK, Branton A, Trivedi D, Nayak G, Panda P, et al. (2016) Determination of isotopic abundance of 13C/12C or 2H/1H and 18O/16O in biofield energy treated 1-chloro-3-nitrobenzene (3-CNB) using gas chromatography-mass spectrometry. Science Journal of Analytical Chemistry 4(4): 42-51.
- Schellekens RC, Stellaard F, Woerdenbag HJ, Frijlink HW, Kosterink JG (2011) Applications of stable isotopes in clinical pharmacology. Br J Clin Pharmacol 72(6): 879-897.
- Weisel CP, Park S, Pyo H, Mohan K, Witz G (2003) Use of stable isotopically labeled benzene to evaluate environmental exposures. J Expo Anal Environ Epidemiol 13(5): 393-402.
- Muccio Z, Jackson GP (2009) Isotope ratio mass spectrometry. Analyst 134(2): 213-222.
- Rosman KJR, Taylor PDP (1998) Isotopic compositions of the elements 1997 (Technical Report). Pure Appl Chem 70(1): 217-235.
- Smith RM (2004) Understanding Mass Spectra: A Basic Approach. (2nd edn), John Wiley & Sons, Inc, New York, USA.
- Jürgen H (2004) Gross Mass Spectrometry: A Textbook. (2nd edn), Springer, Berlin, Germany.
- Sanderson JP, Hollis FJ, Maggs JL, Clarke SE, Naisbitt DJ, et al. (2008) Nonenzymatic formation of a novel hydroxylated sulfamethoxazole derivative in human liver microsomes: Implications for bioanalysis of sulfamethoxazole metabolites. Drug Metab Dispos 36(12): 2424-2428.
- Coplen TB, Böhlke JK, De bièvre P, Ding T, Holden NE, et al. (2002) Isotope-abundance variations of selected elements. Pure and Applied Chemistry 74(10): 1987-2017.
- Wiederhold JG (2015) Metal stable isotope signatures as tracers in environmental geochemistry. Environ Sci Technol 49(5): 2606-2624.
© 2021 Snehasis Jana. This is an open access article distributed under the terms of the Creative Commons Attribution License , which permits unrestricted use, distribution, and build upon your work non-commercially.
 a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in
a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in 







.jpg)






























 Editorial Board Registrations
Editorial Board Registrations Submit your Article
Submit your Article Refer a Friend
Refer a Friend Advertise With Us
Advertise With Us
.jpg)






.jpg)














.bmp)
.jpg)
.png)
.jpg)










.jpg)






.png)

.png)



.png)






