- Submissions

Full Text
Modern Approaches in Drug Designing
Plant Extracts Based Nanoparticles, a Good Perspective in the Development of Drugs in Nanomedicine
Jagessar RC*
Department of Chemistry, South America
*Corresponding author: Jagessar RC, Department of Chemistry, Faculty of Natural Sciences, Guyana, South America
Submission: July 21, 2020;Published: August 14, 2020

ISSN: 2576-9170 Volume3 Issue2
Abstract
Nanotechnology is the design, characterization, production/synthesis and application of structures, devices and systems by controlling the shape and size at the nanometer scale. Nanoparticles are usually synthesized by chemical methods that usually used toxic reactants/reagents as reducing agents that further produce toxic by-products, which in turn are hazardous to the environment. However, recently, there has been the used of plant extracts as an alternative, complementary source of reducing agents to reduce metal ions to the corresponding metal nanoparticles. Plants contain an abundance and diverse arrays of natural products such as alkaloids, flavonoids, saponins, steroids, tannins, coenzymes etc. that vary in concentration and type in different parts of the plants such as leaves, stems, roots, shoots, flowers, barks, fruits and seeds. These secondary metabolites can act as reducing and stabilizing agents for the bio reduction reaction to synthesize novel metallic nanoparticles. Plant based nanoparticles are less expensive to synthesize, ecofriendly and are thus less hazardous to the environment. These plantbased nanoparticles have increasing applications as novel antimicrobial agents in light of the need to find new and alternative antimicrobial agents, as a result of antimicrobial resistance. Also, their efficacy as anticancer and antidiabetic agents are on the research agenda. This presentation on Green nanotechnology, surveys the use of plant extracts, in the synthesis of nanoparticles, incorporating silver, gold, platinum, metal oxides such as TiO2, ZnO and MgO etc, which has medicinal applications.
Keywords: Nanotechnology; Nanoparticles; Plant-based nanoparticles; Natural products; Environmental friendly; Medicinal
Introduction
Nanotechnology, a relatively new area of study and research, is the “design, characterization, production and application of structures, devices and systems, by controlling shape and size at the nanometer scale [1]. The particle matter usually ranges from 1 to 100nm in size. A nanometer (nm) is a billionth of a meter, 10-9. Within this range, materials may have properties considerably different from those expected when they have larger dimensions. Nanoscience depends on the fundamental properties of nano size objects [2,3]. Nanomaterials can show atom-like behaviors, which result from higher surface energy, due to their large surface area. This is in contrast to bulk material which has constant physical properties, regardless of its size. At the nanoscale, this is not often the case [4].
Novel applications of nanoparticles and nanomaterials are expanding rapidly in many frontiers, due to their completely new or enhanced properties, depending on size, their distribution and morphology. Nanotechnology has found applications in many realms. These include health care, medicinal applications such as antibacterial, antiviral, antifungal, anti-inflammatory activities, cosmetics, biomedical, food and feed, drug-gene delivery, environment, health, mechanics, optics, chemical industries, electronics, space industries, energy, science, catalysis, light emitters, single electron transistors, non-linear optical devices and photo-electrochemical applications [5-7].
Metallic nanoparticles have been extensively studied because of their unique physiochemical characteristics such as catalytic activity, opto-electronic properties, antibacterial and magnetic properties [8-12]. Noble metallic NPs, such as Ag, Au, Pd have received increasing interests in the scientific community. [13,14] Their applications are seen in fields such as biomedicine, catalysis, preparation of nanocomposites with tunable electrical conductivity, thermal conductivity, tensile strength, superior rigidity, hardness and erosion resistance, manufacturing of satellite components, aircrafts spares, microchips processors [15-17] etc. The metallic nanoparticles are considered the most promising as they possess remarkable antibacterial properties due to their large surface area to volume ratio which can counteract growing microbial resistance against metal ions, antibiotics, and the development of resistant strains [6]. Of these metallic nanoparticles, silver nanoparticles stand out, because of their unique properties, such as chemical stability, good conductivity, catalytic, antibacterial, anti-viral, antifungal, and anti- inflammatory activities. They are usually found as component of composite fibers, cryogenic superconducting materials, cosmetic products, food industry and in electronic components [18,19]. Ag nanoparticles have found applications in the biomedical field such as in wound dressings, topical creams, antiseptic sprays, and fabrics. Ag nanoparticles display a broad biocidal effect against pathogenic microorganisms via a disruption of their unicellular membrane. Hence, disturbing enzymatic activities. Ag nanoparticles have also been successful in cancer diagnosis and treatment as well [20,21].
Synthesis of Nanoparticles
There are basically, three broad ways of synthesizing metal nanoparticles: chemical, physical, and green synthesis. Chemical and physical methods are quite expensive and potentially hazardous to the environment and involve the use of toxic and perilous chemicals that are responsible for the various biological and environmental risks. Physical synthetic methods include inert gas condensation, severe plastic deformation, high energy ball milling and ultrasonic shot peeling [22] Commonly grinding process and pyrolysis can be used. However, physical synthesis has not been reliable to obtain metallic nanoparticles. The particles are usually larger than 100nm which is not nanometer size. Physical methods are expensive and cumbersome for large scale production of nanoparticles [23]. In addition, “physical approaches” require enormous consumption of energy to maintain the high pressure and temperature used in the synthesis.
Chemical synthesis of nanoparticles involves the reduction of chemicals [24], electrochemical methods [25], reduction of phytochemicals [26], microemulsion, chemical coprecipitation, chemical vapor condensation and pulse electrodeposition. A typical nano- chemical procedure involves growing nanoparticles in a liquid medium containing various reactants, in particular reducing agents such as sodium borohydride or potassium bitartrate or methoxy polyethylene glycol or hydrazine [27,28]. To prevent the agglomeration of metallic nanoparticles, a stabilizing agent such as sodium dodecyl benzyl sulphate or polyvinyl pyrrolidone is added to the reaction mixture. Hence, chemical methods used for the synthesis of the nanoparticles are too expensive and involved the use of toxic and hazardous chemicals that are responsible for environmental and biological risks.
Chemically, nanoparticle can be classified as “bottom up” and “top down” by the direction of nanoparticle formation, Figure 1. The bottom up reaction begins from the atomic level, through forming molecules. In the top down approach, the scale of the resultant nanoparticles is larger, so that a mechanical process or the addition of acids is required to reduce the particle size. The “top down” technique requires the use of complex and complicated instrumentation [29]. Thus, its necessary to use safer (environmental and biological) and cost-effective methods to synthesize metallic nanoparticles. This has given birth to the synthesis of nanoparticles using “Green Chemistry”. The advancement of “Green Chemistry” over chemical and physical methods, for the synthesis of metallic nanoparticles is environmentally, biologically friendly. Also, its cost effective, and can be scaled up for large scale synthesis of nanoparticles. In addition, there is no need to use high temperature, pressure, energy, and toxic chemicals [30]. Development of plant-based nanoparticles has many advantages over conventional physio-chemical methods and has various applications in medicine and biology. For the synthesis of plant-based nanoparticles, the following are necessary:
a) metal salt,
b) a reducing agent, and
c) a stabilizing or capping agent for controlling the size of nanoparticles and preventing their aggregation [31].
Table 1: Some plants, whose extracts have been used for the synthesis of NPS
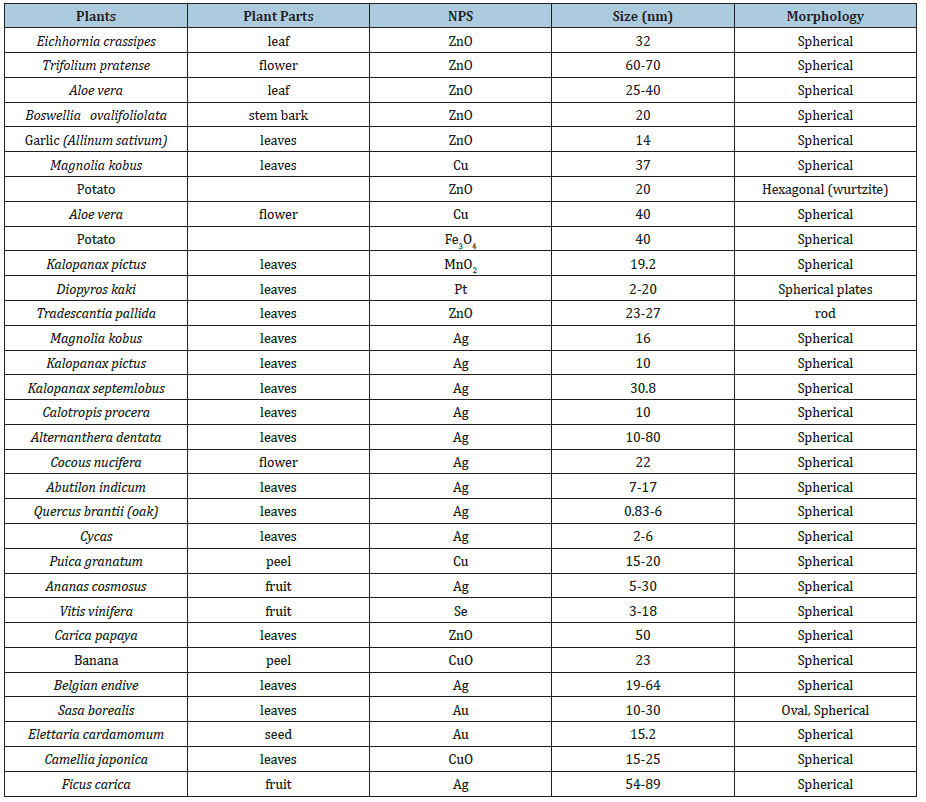
Figure 1: The synthesis of nanoparticles via the “top to bottom” and “bottom to up” approach.
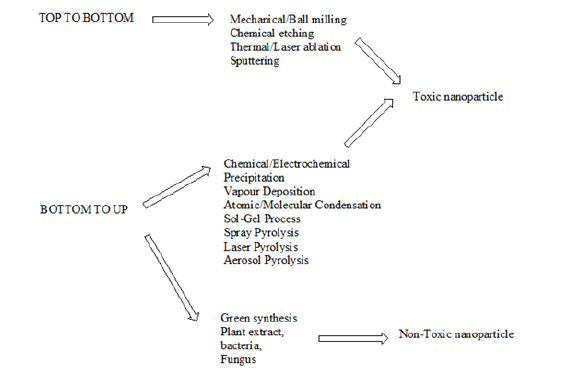
Plant parts such as leaves, fruits, seeds, stems, flowers, roots, barks, and fruit peels are involved in the synthesis of various types of nanoparticles. Table 1 shows some selected plants used in the formation of plant-based nanoparticles, PBNP. Nanoparticles such as silver from silver nitrate, gold from gold chloride, zinc oxide from zinc nitrate and zinc acetate, cadmium sulfide and zinc sulfide from cadmium sulfate and zinc sulfate, respectively, are synthesized with the help of different types of plants and their different parts are reported. Figure 2 shows a possible mechanism of formation of Cu and ZnO NPs by using extract of plants. Figure 3 shows a possible mechanism of formation of Cu and ZnO NPs by using extract of plants, whereas Figure 4 shows some natural products responsible for the formation of plant based natural products.
Figure 2: Possible mechanism of formation of Cu and ZnO NPs by using extract of plants.
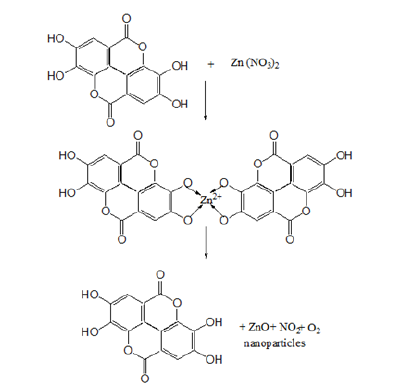
Figure 3: Protocol for the synthesis of plant-based nanoparticles.
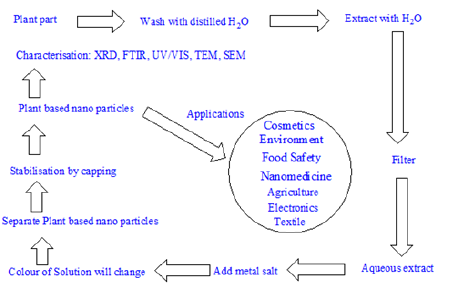
Characterization of Plant Based Nanoparticles
Whatever is the route of synthesis, PBNP are usually characterized via the following techniques: UV-visible spectrophotometry, Powder X-ray diffraction (XRD), dynamic light scattering (DLS), Fourier transform infrared spectroscopy (FTIR), scanning electron microscopy (SEM), transmission electron microscopy (TEM), and energy dispersive spectroscopy (EDS).
Application of Nanotechnology
Plant based nanoparticles have found diverse applications in medicine (nanomedicine). For example, plant-based silver nanoparticles are well known for their antimicrobial activities, especially for their antifungal, antiviral and anti-inflammatory activities. It has been found that silver nanoparticles inactivate microbes by interacting with microbial enzymes, proteins or DNA and so prevent multiplication [32]. Silver nanoparticles were also found to be highly active against human immunodeficiency virus [33]. Fungicidal effects of biosynthesized metal nanoparticles such as silver have more potential than the commercially available antibiotics, such as amphotericin and fluconazole. They showed membrane damage in fungal intracellular components and then finally lead to the death of fungal cells [34].
Plant latex capped silver nanoparticles was tested against human lung carcinoma cells, and it was found that these silver nanoparticles are toxic to –AS49 cells in a dose dependent manner. Plant latex can act as a stabilizer for the silver nanoparticles in water and can also be responsible for the transportation of nanoparticles to the target cells [35]. It is reported that gold and platinum plant-based nanoparticles have significant positive wound healing mechanism and tissue regeneration through anti-inflammatory functions. Anti-inflammatory effect is important for wound healing mechanism. It produces compounds such as cytokines and interleukins, which is produced by specific T-lymphocytes, B-lymphocytes, and macrophages [36]. Plant based nanoparticles have also found application as antioxidant entity. Antioxidant agents include mainly enzymatic and non-enzymatic compounds that can regulate the formation of free radicals. These free radicals are found to be responsible for inducing cellular damage such as brain, cancer, and atherosclerosis. Antioxidant efficiency of silver nanoparticles have been found to be much higher than that of other synthetic compounds such as ascorbic acid etc. [37].
Figure 4: Some natural products responsible for the formation of plant based natural products.

Plant based nanoparticles can be used for antiviral treatments. Viruses enter their host very rapidly and multiply quickly. Plant based silver nanoparticles can act as potent antiviral agent for a wide range of viral infections. Plant based silver nano particles have been found to be effective against HIV pathogens [38]. Plants have been used as traditional sources for the development of drugs for antimalarial diseases. Plant derived chemicals such as quinine, artemisinin and aromatic compounds are effectively and efficiently used against malarial parasites. It has been found that plant-derived nanoparticles from metals, such as gold, platinum, palladium, and silver are used for the effective control of malarial population in the environment. The use of plant-based nanoparticles as an anti- plasmodial agent is found to be more economic, but less effective to control the target organism in health centers [39]. Plant based nanoparticles, especially gold have found application in the management of diabetes mellitus (DM). Research has shown that gold nanoparticles significantly reduce the alkaline phosphatase, uric acid, and serum creatinine in treated mice [40].
There has been an increasing number of research studies on the synthesis of nanoparticles using plant extracts. These syntheses make use of microorganisms, including bacteria, fungi, and plants, because of their antioxidant or reducing properties, responsible for the reduction of metal compounds in their respective nanoparticles. Microbes mediated synthesis of nanoparticles is not of industrial feasibility due to the requirements for highly aseptic conditions and their maintenance. Hence, the use of plant extracts for the synthesis of nanoparticles is potentially advantageous over microorganisms, due to the ease of improvements, the less biohazard, and the elaborate process of maintaining cell cultures [41].
A rapid, simple approach was applied for the synthesis of silver nanoparticles using Azadirachta indica aqueous leaf extract. The plant extract performs its role as both a reducing agent, as well as a capping agent. The functional groups present in the plant, responsible for the reduction of silver ions were investigated by FTIR. The synthesized nanoparticles were characterized via DSL, photoluminescence, TEM and UV-Visible spectrophotometer. UV-Visible spectrophotometer showed absorbance peak in the range 436-446nm. The silver nanoparticles displayed antibacterial activities against both gram positive (Staphylococcus aureus) and gram negative (Escherichia coli) microorganisms. Only 15 minutes were, required for the conversion of silver ions into silver nanoparticles at room temperature without the need for the involvement of any hazardous chemical, thus confirming a simple, rapid, one-step, ecofriendly, nontoxic alternative to conventional physical/chemical methods [42].
Plant parts such as leaves, fruits, seeds, stems, flowers, roots, barks, and fruit peels are involved in the synthesis of various types of nanoparticles. The low cost and high-eco-friendly-natured plants are very advanced and beneficial to human applications. Nanoparticles such as silver from silver nitrate, gold from gold chloride, zinc oxide from zinc nitrate and zinc acetate, cadmium sulfide and zinc sulfide from cadmium sulfate and zinc sulfate, etc. were synthesized with the help of different types of plants and their different parts are reported [43]. Gold nanoparticles (GNPs) were prepared via four different plant extracts as reducing and stabilizing agents. The extracts were obtained from the following plants: Salvia officinalis, Lippia citriodora, Pelargonium graveolens and Punica granatum. The size distributions of the GNPs were measured using three different methods: dynamic light scattering, nanoparticle-tracking analysis, and analysis of scanning electron microscopy images. The three methods yielded similar size distributions. High-resolution transmission electron microscopy was used to view the shapes of the larger GNPs, while infrared spectroscopy was used to characterize the various functional groups in the organic layer that stabilize the particles. Biocompatibility was examined by correlation of L-cell growth in the presence of different amounts of GNPs. All GNPs showed good biocompatibility and good stability for over 3 weeks. Therefore, they can be used for imaging and drug-delivery applications in the human body. Its postulated that the antioxidants in the plant extracts might be involved in the formation of GNPs [44].
A simple and rapid synthesis of silver nanoparticles (AgNPs) using an aqueous leaf extract of Alysicarpus monilifer and its antibacterial efficacy against multi‐drug‐resistant MRSA and CoNS isolates from HIV patients has been reported49. The green‐synthesized AgNPs were characterized using ultraviolet‐visible spectroscopy, transmission electron microscopy, energy dispersive X‐ray analysis, selected area electron diffraction pattern, X‐ray diffraction patterns, and Fourier transform infrared spectroscopy. Stable, welldefined AgNPs are mostly spherical in shape with a mean size of 15 ± 2nm, were obtained within an hour. Green synthesized AgNPs revealed significant dose‐dependent antibacterial action against MRSA and CoNS isolates. Biogenic AgNPs have demonstrated to be potent antibacterial agents in comparison with conventional antibiotics. The emergence of multi‐drug‐resistant microorganisms in hospital environments is a global public health problem and are a threat to everyone, especially HIV‐infected patients. Methicillinresistant Staphylococcus aureus (MRSA) and coagulase‐negative Staphylococci aureus and (CoNS) are the major causative agents associated with morbidity and mortality in HIV patients. Therefore, control of MRSA and CoNS‐related infections in HIV patients is a worldwide concern and there is a need to develop medicines [45].
The synthesis of ZnO plant-based nanoparticles have been reported. In this present study, zinc oxide (ZnO) nanoparticles (NPs) were synthesized using leaf extracts of two medicinal plants Cassia fistula and Melia azadarach. 0.01M zinc acetate dihydrate was used as a precursor in leaf extracts of respective plants for NPs synthesis. The structural and optical properties of NPs were investigated by X-ray diffraction (XRD), Fourier transform infrared (FTIR) spectroscopy, scanning electron microscope (SEM), ultraviolet-visible spectrophotometer (UV-Vis) and dynamic light scattering (DLS). UV peaks at 320nm and 324nm, and XRD pattern matching confirmed the presence of pure ZnO NPs. FTIR further confirmed the presence of bioactive functional groups involved in the reduction of bulk zinc acetate to ZnO NPs. SEM analysis displayed the shape of NPs to be spherical, whereas DLS showed their size, ranging from 3 to 68m. The antibacterial potential of ZnO NPs was examined by paper disc diffusion method against E. coli and S. aureus, based on the zone of inhibition and minimal inhibitory indices (MIC). Change in color of the reaction mixture from brown to white indicated the formation of ZnO NPs. The C. fistula and M. azadarach, mediated ZnO NPs, showed strong antimicrobial activity against clinical pathogens, compared to standard drugs, suggesting that plant-based synthesis of NPs can be an excellent strategy to develop versatile and eco-friendly biomedical products [46].
Plant-mediated synthesis of silver (Ag) nanoparticles, AgNPs, is noted in the literature using aqueous extracts of fresh leaves of Impatiens balsamina and Lantana camara medicinal plants as bio reducing agents. The synthesis of the nanoparticles was confirmed by ultraviolet-visible (UV-Vis) spectrophotometry and transmission electron microscopy (TEM). UV-Vis spectra and visual observation showed that the color of the fresh leaf extracts of L. camara and I. balsamina turned into grayish brown and brownish yellow, respectively, after treatment with Ag precursors. In addition, TEM analysis confirmed that AgNO3 solutions for all concentrations produced Ag nanoparticles and their average size was less than 24nm. Moreover, aqueous leaf extracts of I. balsamina and L. camara were separately tested for their antimicrobial activity against Gram-positive Staphylococcus aureus and Gram-negative Escherichia coli bacteria. The results showed that the bacterial growth was inhibited by the extracts containing silver (Ag) nanoparticles. Statistical calculation performed using the Tukey test showed that zones of inhibition for the two bacteria produced by the aqueous leaf extracts of L. camara, containing 3mM and 5mM Ag precursors, were not significantly different from that by ciprofloxacin as the positive control. On the contrary, there was a significant difference between the zone of inhibition for E. coli by ciprofloxacin and that by the extracts of I. balsamina leaves containing 3mM and 5mM Ag precursors. A similar result was observed on the zone of inhibition for S. aureus by the extracts of I. balsamina leaves containing 3mM Ag precursor. It was shown that the aqueous extracts of fresh L. camara leaves containing silver (Ag) nanoparticles, AgNPs were comparable to ciprofloxacin in inhibiting bacterial growth [47].
Conclusion
There are three ways to make nanoparticles, physical, chemical and “green synthesis. From the preceding discussion, it can be envisaged that “green” synthesis is the best since its very simple, easy to perform, inexpensive, highly efficient, and environmentally friendly. It is the chemical constituents of plants, proteins, carbohydrates, alkaloids, tannins, phenolics, oils and saponins, which apart from their medicinal uses can act as reducing and capping agents for NP synthesis. The shape and size distribution of plant-based NP can be controlled by an optimization of reaction conditions, such as temperature, pH, and the amount of plant material. Besides, the reaction can be scale up.
References
- (2004) Nanoscience and nanotechnologies. The Royal Society & The Royal Academy of Engineering.
- Abou El-Nour KMM, Eftaiha Aa, Al-Warthan A, Ammar RAA (2010) Synthesis and applications of silver nanoparticles. Arabian Journal of Chemistry 3 (3): 135-140.
- Mohanpuria P, Rana NK, Yadav SK (2007) Biosynthesis of nanoparticles: Technological concepts and future applications. Journal of Nanoparticle Research 10 (3): 507-517.
- Van Dijken A, Meulenkamp EA, Vanmaekelbergh D, Meijerink A (2000) Identification of the transition responsible for the visible emission in ZnO using quantum size effects. Journal of Luminescence 90(3-4): 123-128.
- Korbekandi H, Iravani S (2012) Silver nanoparticles, the delivery of nanoparticles. In: Hashim Abbass A (Ed.), Silver Nanoparticles.
- Khalil KA, Fouad H, Elsarnagawy T, Almajhdi FN (2013) Preparation and characterization of electrospun PLGA/silver composite nanofibres for biomedical applications. Int J Electrochem Sci 8: 3483-3493.
- Kaviya SSJ, Viswanathan B (2011) Green synthesis of silver nanoparticles using Polyalthia longifolia leaf extract, along with D-sorbitol. J Nanotech 201: 1-5.
- Krolikowska A, Kudelski A, Michota A, Bukowska J (2003) SERS studies on the structure of thioglycolic acid monolayers on silver and gold. Surface Science 532-535: 227-232.
- Crabtree JH, Burchette RJ, Siddiqi RA, Huen IT, Hadnott LL, et al. (2003) The efficacy of silver-ion implanted catheters in reducing peritoneal dialysis-related infections. Peritoneal Dialysis International 23(4): 368-374.
- Catauro M, Raucci MG, de Gaetano F, Marotta A (2004) Antibacterial and bioactive silver -containing Na2 CaO.2SiO2 glass prepared by sol-gel method. Journal of Materials Science: Materials in Medicine 15(7): 831-837.
- Fardood ST, Ramazani A, Moradi S (2017) Green synthesis of Ni-Cu-Mg ferrite nanoparticles using tragacanth gum and their use as an efficient catalyst for the synthesis of polyhydroquinoline derivatives. Journal of Sol-Gel Science and Technology 8(2): 432-439.
- Fardood S, Ramazani A, Golfar Z, Joo SW (2017) Green synthesis of Ni-Cu-Zn ferrite nanoparticles using tragacanth gum and their use as an efficient catalyst for the synthesis of polyhydroquinoline derivatives. Applied Organometallic Chemistry 31(12): e3823.
- Arvizo RR, Bhattacharyya S, Kudgus RA, Giri K, Bhattacharya R, et al. (2012) Intrinsic therapeutic applications of noble metal nanoparticles: Past, present and future. Chemical Society Reviews 41(7): 2943.
- Beitollai H, Zaimbashi R (2017) A new sensor based on graphite screen printed electrode modified with Cu-nanocomplex for determination of paracetamol. Nanochemistry Research 2(1): 151-158.
- Issa B, Obaidat J, Albiss B, Haik Y (2013) Magnetic nanoparticles surface effects and properties related to biomedicine applications. International Journal of Molecular Sciences 14(11): 21266-21305.
- RaO CNR, Ramakrishna Matte HSS, Voggu R, Govindaraj A (2012) Recent progress in the synthesis of inorganic nanoparticles. Dalton Transactions 41(17): 5089.
- Zeiri Y, Elia P, Zach R, Hazan S, Kolusheva S, et al. (2014) Green synthesis of gold nanoparticles using plant extracts as reducing agents. International Journal of Nanomedicine 9: 4007.
- Ahmad A, Mukherjee P, Senapati S, Mandal D, Khan MI, et al. (2003) Extracellular biosynthesis of silver nanoparticles using the fungus Fusarium oxysporium. Colloids Surf B: Biointerfaces 28(4): 313-318.
- Klaus-Joerger T, Joerger R, Olsson E, Granqvist C (2001) Bacteria as workers in the living factory: Metal accumulating bacteria and their potential for material science. Trends Biotechnol 19(1): 15-20.
- Popescu M, Velea A, Lorinezi A (2010) Biogenic production of nanoparticles. Dig J Nano Nanomater Bios 5(4): 1035-1040.
- Baruwati B, Polshettiwar V, Varma RS (2009) Glutathione promoted expeditious green synthesis of silver nanoparticles in wáter using microwaves. Green Chem 11(7): 926-930.
- Li X, Zhang W (2006) Iron nanoparticles: The core-shell structure and unique properties for Ni (II) sequestration. Langmuir 22(10): 4638-4642.
- Li Y, Duan X, Qian Y, Yang L, Liao H (1999) Nanocrystalline silver particles: Synthesis, agglomeration and sputtering induced by electron beam. Journal of Colloid and Interface Science 209(2): 347-349.
- Maribel G, Guzman JD, Stephan G (2009) Synthesis of silver nanoparticles by chemical reduction method and their antibacterial activity. International Journal of Chemical and Biomolecular Engineering 2: 104.
- Rodriguez-Sanchez L, Blanco MC, Lopez-Quintela MA (2000) Electrochemical synthesis of silver nanoparticles. The Journal of Physical Chemistry B 104(41): 9683-9688.
- Sharma VK, Yngard RA, Lin Y (2009) Silver nanoparticles. Green synthesis and their antimicrobial activities. Advances in Colloid and Interface Science 145(1-2): 83-96.
- Tan Y, Dai X, Li Y, Zhu D (2003) Preparation of gold, platinum, palladium and silver nanoparticles by the reduction of their salts with a weak reductant-potassium bitartrate. Journal of Materials Chemistry 13(5): 1069-1075.
- Mallick K, Witcomb MJ, Scurrell MS (2004) Polymer stabilized silver nanoparticles: A photochemical synthesis route. Journal of Materials Science 39(14): 4459-4463.
- Raspolli GAM, Antonetti C, Marracci M, Piccinelli F, Tellini B (2013) Novel microwave synthesis of Cu nanoparticles in the absence of any stabilizing agent and their antibacterial and antistatic applications. Applied Surface Science 280(1): 610-618.
- Dhuper S, Panda D, Nayak PL (2012) Green synthesis and characterization of zero valent iron nanoparticles from the leaf extract of Mangifera indica. Nano Trends: J Nanotech App 13(2): 16-22.
- Ledwith DM, Whelan AM, Kelly JM (2007) A rapid, straight forward method for controlling the morphology of stable nanoparticles. Journal of Materials Chemistry 17(23): 2459.
- Tanaka K (1999) Nanotechnology towards the 21st Thin Solid Films 34(1-2): 120-125.
- Elechiguerra JL, Burt JL, Morones JR, Camacho-Bragado A, GaO X, et al. (2005) Interaction of silver nanoparticles with HIV-1. J Nanobiotech 3: 6.
- Logeswari P, Silambarasan S, Abraham J (2012) Synthesis of silver nanoparticles using plant extracts and analysis of their antimicrobial activity. J Saudi Chem Soc 4: 23-45.
- Song JY, Jang HK, Kim BS (2099) Biological synthesis of gold nanoparticles using Magnola Kobus and Diopyros kaki leaf extracts. Process Biochem 44(10): 1133-1138.
- Jacob SJ, Finub JS, Narayanan A (2012) Synthesis of silver nanoparticles using Piper langum leaf extracts and its cytotoxic activity against Hep-2 cell line. Colloids Surf B 91: 212-214.
- Abdel-Aziz, MS, Shaheen MS, El-Nekeety AA, Abdel Wahhab MA (2014) Antioxidant and antibacterial activity of silver nanoparticles biosynthesized using Chenopodium murale leaf extract. J Saudi Chem Soc 18(4): 356-363.
- Suriyakalaa U, Antony JJ, Suganya S, Siva D, Sukirtha R, et al. (2013) Hepatocurative activity of biosynthesized silver nanoparticles fabricated using Andrographis paniculata. Colloids Surf B 102: 189-194.
- Jayaseelan C, Rahuman AA, Rajakumar G, Kirthi AV, Santhoshkumar T, et al. (2011) Synthesis of pediculocidal and larvicidal silver nanoparticles by leaf extract from heartleaf moonseed plant, Tinospora cordifolia Parasitol Res 109(1): 185-194.
- Daisy P, Saipriya K (2012) Biochemical analysis of Cassia fistula aqueous extract and phytochemically synthesized gold nanoparticles as hypoglycemic treatment for diabtes mellitus. Int J Nanomed 7: 1189-1202.
- Kalishwaralal K, Deepak V, Pandian RK, Kottaisamy BSM, Kartikeyan KS, et al. (2010) Biosynthesis of silver and gold nanoparticles using Brevibacterium casei. Colloids Surf B: Biointerfaces 77(2): 257-262.
- Ahmed S, Saifullah MA, Swami BL, Ikram S (2016) Green synthesis of silver nanoparticles, using Azadirachta indica aqueous leaf extract. Journal of radiation Research and Applied Sciences 9(1): 1-7.
- Tripathi DK, Ahmad P, Dubey NK (2018) Nanomaterials in plants, algae, and microorganisms. Concepts and Controversies, Volume 1.
- Elia P, Zach R, Hazan S, Kolusheva S, Porat Z, et al. (2014) Green synthesis of gold nanoparticles using plant extracts as reducing agents. Int J Nanomedicine 9: 4007-4021.
- Kasithevar M, Saravanan, M, Prakash P, Kumar H, Ovais M, et al. (2017) Green synthesis of silver nanoparticles using Alysicarpus monilifer leaf extract and its antibacterial activity against MRSA and CoNS isolates in HIV patients. Journal of Interdisciplinary Nanomedicine 2(2): 134.
- Naseer M, Aslam U, Khalid B, Chen B (2020) Green route to synthesize zinc oxide nanoparticles using leaf extracts of Cassia fistula and Melia azadarach and their antibacterial potential. Scientific Reports 10(9055): 1-10.
- Aritonang HF, Koleangan H, Wuntu AD (2019) Synthesis of silver nanoparticles using aqueous extract of medicinal plants’ (Impatiens balsamina and Lantana camara) fresh leaves and analysis of antimicrobial activity. International Journal of Microbiology, pp. 1-8.
© 2020 Jagessar RC. This is an open access article distributed under the terms of the Creative Commons Attribution License , which permits unrestricted use, distribution, and build upon your work non-commercially.
 a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in
a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in 







.jpg)






























 Editorial Board Registrations
Editorial Board Registrations Submit your Article
Submit your Article Refer a Friend
Refer a Friend Advertise With Us
Advertise With Us
.jpg)






.jpg)














.bmp)
.jpg)
.png)
.jpg)










.jpg)






.png)

.png)



.png)






