- Submissions

Full Text
Modern Approaches in Drug Designing
Recent Insights into β-Carboline Alkaloids with Anticancer Potential
Lakshmi Manasa K, Swetha Yadav S, Srikanth D, Narayana Nagesh* and Mallika Alvalaa
1Department of Medicinal Chemistry, India
2CSIR-Centre for Cellular and Molecular Biology, India
*Corresponding author: Narayana Nagesh, CSIR-Centre for Cellular and Molecular Biology, India
Submission: June 05, 2020;Published: July 31, 2020

ISSN: 2576-9170 Volume3 Issue1
Abstract
Development of nature inspired new drug molecules through medicinal chemistry approach has a profound success on drug discovery. Efforts of chemists have succeeded in developing semi- synthetic derivatives to dominate the authentic natural product in terms of drug-likeness properties include increased potency, reduced toxicity, and patient compliance. β-carbolines a family of indole-based alkaloids, remained as a privileged scaffold to exhibit anticancer effects through numerous mechanisms. This review inclines the readers towards the ongoing developments (2017-2019) on cytotoxic effects of β-Carboline with a significance on structure based rational drug design, multiparameter lead optimization strategies, relevant SAR studies of this particular framework.
Keywords: β-Carbolines; FaβCs; DHβCs; THβCs; Cytotoxic agents; Topoisomerase inhibition activity; Docking studies
Abbreviations: FaβCs: Fully Aromatic Carbolines; DHβCs: Dihydro Carbolines; THβCs: Tetrahydro-β- Carbolines, CDK: Cyclin-Dependent Kinases, PLK: Polo-Like Kinases; PARP: Poly (ADP-Ribose) Polymerase
Introduction
Natural products have a protracted history as therapeutics for broad range of diseases. Indelible co-evolution between biological communities and humans has tried to explain the baffle of biological significance of natural products in humans and other species [1-7]. Many chemists and biologists in both industrial and academic sector have commenced and proved clinical potentiality of natural compounds as a prolific source of chemical inspiration for the evolution of new drugs. The impact of plant-derived drugs on mankind become enormous in the recent days and is proved by the development of plant-derived drugs such as vinblastine, vincristine, paclitaxel, quinine, etoposide, artemisinin, teniposide, morphine, and the camptothecin derivatives topotecan and irinotecan. Even though, natural products derived from microbial origin have made significant contribution, marine derived natural products are also having an increasing impact on the treatment of human disease, particularly as anticancer agents [8-10]. Evolution of semi-synthetic modifications of natural products as a source of bioactive-lead compounds to improve drug-likeness and clinical utility is one of the transitions taken an advanced role in drug discovery and drug development. Hence, further research regarding the development of new chemotherapeutic agents that are more effectively combat cancer is an active area of research in medicinal chemistry [11-13].
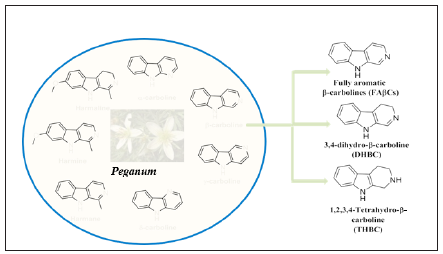
Carbolines are nature-derived heterocyclic compounds containing indole ring fused with pyridine (fused benzene-pyrrole-pyridine system) system [14]. Carbolines were first found in harmala alkaloids and were found to be widespread in both plant as well as animals. Carbolines are classified based on the position of nitrogen on pyridine ring as α-, β-, γ- and δ- carbolines (Figure1) [15]. Among all the carbolines, β-carbolines have been observed as major-stock holder, due to their dynamic use in the treatment of various diseases including psychopharmacological and oncological properties [16]. β-Carbolines are a group of alkaloids having a planar tricyclic pyrido [3,4-b] indole ring system [17] and are originally isolated from seeds of Peganum harmala; Zygophillaceae family and has been used traditionally for the treatment of alimentary tract cancers and malaria [18]. These are widely distributed in plant (leaves, barks and roots), microorganisms, insects, marine invertebrates (bryozoans, hydroids, soft corals, sponges), marine ascidians (genus Eudistoma) [17], mammalians (human tissues and body fluids like blood, cerebro-spinal fluid, etc.) [19], various food products (tomatoes, kiwi, fruit juice, fish, grilled bacon, etc.) [20], coffee, alcoholic beverages and tobacco smoke [21]. These exhibits various pharmacological properties include anticonvulsant, antifungal, antimicrobial, antiviral, antiplasmodial, antiparkinson, antialzheimer, anxiolytic and antitumor property [22-28].
Figure 1: β-carbolines and their derivatives
The best-known natural products which contain β-carboline skeleton include norharmane, harmine, harmane and harmaline. Harmine type of β- carbolines are found to possess profound anticancer effects through multiple mechanisms such as inhibition of CDK’s [29,30], topoisomerase I & II [31], MK-2 [32], PLKs [33,34], DYRK1A [35,36] and DNA intercalation or binding through minor groove [37]. Besides, Harmane like β -carbolines interact with multiple neuro-receptors, such as that of serotonin, dopamine, benzodiazepine, opiate, nicotine, histamine and imidazoline binding sites (I-BS) and thus mediate numerous psychopharmacological effects. β-carboline alkaloids are isolated from many other plants.
Classification of β-Carbolines
β-carbolines are further classified based on the saturation of the N-containing 6-membered ring (pyridine ring). Unsaturated pyridine ring containing compounds (Figure 1) are named as Fully aromatic Beta-Carbolines (FAβCs), partially and fully saturated compounds are named as 3,4- dihydro-β-carbolines (DHβCs) and 1,2,3,4-tetra hydro-β-carbolines (THβCs) respectively.
A. FAβCs: A large group of natural and synthetic indole alkaloids that possess a common tricyclic pyrido [3,4-b] indole ring with unsaturated pyridine ring system. These derivatives were generally synthesized via two-step process in a stepwise fashion.
B. Generally synthesized by Pictet-Spengler reaction followed by in situ decarboxylation and then aromatization [38,39].
C. DHβCs: These types of alkaloids possess a tricyclic pyrido[3,4-b] indole ring as common but with partially saturated pyridine ring system, hence called as 3,4-dihydro-β-carbolines (DHβCs). These can be synthesized via Pictet-Spengler reaction followed by dehydrogenation [40].
D. THβCs: These tricyclic systems usually contain saturated pyridine ring in the tricyclic pyrido[3,4-b] indole ring system the most traditional methods to synthesize THβC frameworks are Pictet-Spengler and Bischler-Napieralski reaction. Among the huge number of β-carbolines, THβCs found to present in large number of natural products and exhibit different biological properties [41] (Table 1).
Table 1: List of some natural β-carboline derivatives.

β-Carboline and its Derivatives
Triazole-β-carboline derivatives
Abdelsalam et al. [92] have reported a series of 24 novel 1-(3-hydroxyphenyl)-9H-β-carboline (Figure 2) possessing oxadiazoles and triazoles at C3-position and assayed against various cancer cell lines. Replacement of 1,3,4-oxadiazole with its bioisostere N4-substituted-1,2,4-triazole moiety enhanced the cytotoxic activity. Moreover, the presence of 4-tolyl substituent on 1,2,4-triazole moiety showed potent anticancer activity. Further retention of cytotoxic activity by the S- methylation of the sulfanyl group. S-alkylation using bulkier groups such as ethoxycarbonyl methylene or 4-substituted phenacyl moieties dramatically decreased the antitumor activity. Compound 1 was found to be potent among the series. Further mechanistic studies demonstrated that compound 1 elicits sub-G1 apoptosis and arrest the cell cycle at G2/M phase in MDA-MB- 435 cells. In silico physicochemical and ADME parameters revealed that potent compounds have acceptable bioavailability and pharmacokinetic parameters upon oral administration. Also, authors reported the binding affinity of compound 1 with topo-I and KSP ATPase. Thus, this study revealed a potential lead for the topo I and KSP ATPase inhibitors [92].
Figure 2: β-carboline-linked 1,2,4-triazole as cytotoxic agents.

Triazole-tetrahydro-β-carboline derivatives
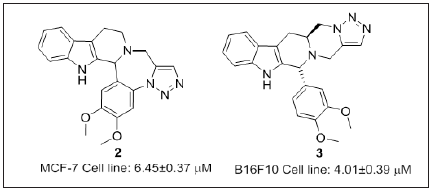
Shankaraiah et al. [93] has reported a series of 1,2,3-Triazololinked- tetrahydro-β-carboline derivatives (Figure 3) via intramoecular 1,3-Dipolar cycloaddition reaction. Compound 2 and 3 having free indole NH and electron donating group substituted at C6 phenyl ring showed potent cytotoxicity. Thus, polyheterocyclic annulated molecules displayed synergistic mechanism of action.
Figure 3: Tetrahydro-β-carboline-linked 1,,3-triazoles derivatives.
1,3,4-oxadiazole-β-carboline derivative
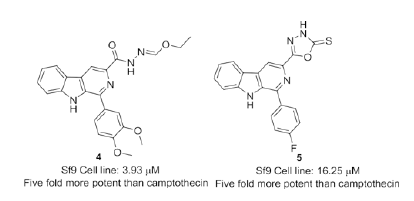
Zhong et al. [94] disclosed a series of insect growth inhibitors by combining the core pharmacophore β-carboline with 1,3,4-oxadiazole and tested against Sf9 cells. SAR analysis revealed that substitution at C2-position on oxadiazole motif and electron withdrawing groups at C1-β- carbolines were crucial for activity. Compound 4 and 5 (Figure 4) were found to be fivefold more potent than standard molecule camptothecin via activating Sfcaspase- 1 and significantly inhibit the growth of larvae of S. litura in vivo. Further these compounds can serve as a potential lead in the development of insect growth regulators.
Figure 4: β-carboline-linked 1,3,4-oxadiazoles as insect growth regulators.
Acyl hydrazone-β-carboline derivative
Compound 6 a novel β-carboline/acyl hydrazone (Figure 5) based antitumor agent has been reported by Chen et al. [95] was shown to be active against resistant cancer cell lines and inhibited tumor growth with low side effects, toxicity, without significant loss of body wt. Compound 6 showed drug resistance index low when compared to the standard colchicines, paclitaxel, vinblastine and adriamycin. Further studies have undergone on nude mice to monitor the antitumor effects on H460 xenograft model. Therefore, acylhydrazones which can be further explored to improve the solubility and biological activity (Figure 6).
Figure 5: β-carboline based acylhydrazones.
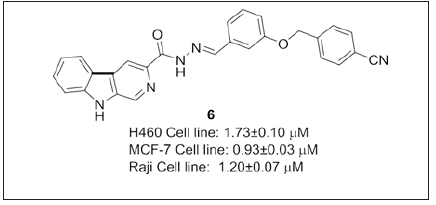
Figure 6: SP141based derivatives.

Naphthalene-β-carboline derivative
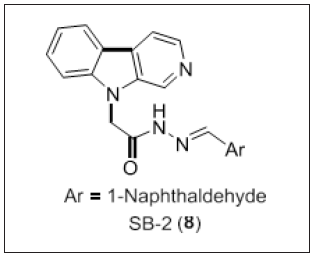
Zhang et al. [96] developed SP141, a dual target molecule for cancer therapy. SP141 (β-carboline derivative) exerts its effect activity by directly bounding to β-catenin. Therefore, the authors disclosed SP141 as a potential scaffold having dual inhibitory activity on β-catenin and MDM2. Chandrasekar et al. [97] have reported a series of N9-substituted β-Carbolines (Figure 7) as PLK- 1inhibitors. SAR studies disclosed that cytotoxic activity was more prominent in β-carboline moiety substituted with naphthalene as well as indole rings. The order of reactivity towards cytotoxic potential was naphthalene > indole > 6-membered heterocyclic > 5-membered heterocyclic rings. Compound 8 was found to be most potent with a GI50 3-45μM on NCI-60 panel cancer cell lines and selectively inhibits PLK-1 at 15μM. It arrests the cell cycle at S/G2 phase on HCT-116 cell line and induced apoptosis by the activation of procaspase-3 and cleaved PARP. SB-2 subjected to in vivo models and considerably increased their average lifespan. In silico studies revealed that inhibition of PLK-1 was due to the interaction between SB-2 and unusual residues, Arg136 and Leu132 present in the hinge region of PLK-1 protein.
Figure 7: N9-substituted β-Carbolines as PLK-1 inhibitors.
β-carboline dimers
Wang et al. [20] disclosed the synthesis and structure activity relationship of bivalent β-carboline derivatives modified at the N9 position and dimerized at the C3-position. Compound 9 (Figure 8) was found to be most potent anticancer compound with an IC50 value 5.61μM. Study revealed that dimers with linker size four to six methylene units were more active compared to monomers, concluding that influence of size of the linker for antitumor activity. Also demonstrated the enhanced antitumor activity by the modification of the β-carboline structure (i.e, from monomer to dimer). Compound 9 could serve as a lead molecule for the development of potential DNA intercalating agents. Dai et al. [98] have reported compound 10 and 11 a novel N9- heterobivalent β-carbolines (Figure 9) with an IC50 value 8.4 and 14.1μM respectively against MCF-7 cell line. In vivo studies were performed for the compound 10 and 11 against mice, with tumor inhibition rate 40% bearing Sarcoma 180 and Lewis lung cancer. Compound 10 also reported for angiogenetic activity and was more potent compared to its standard CA4P. SAR studies revealed that C1 methylation and C7 methoxylation are more favorable to enhance the activity. 3-Pyridyl or 2-thienyl group at C1- position of β-carboline core and aryl substitution at another β-carboline ring can reduced the cytotoxic activity. Structural modification studies of N9-heterodimeric β-carbolines would serve for designing most potent compounds.
Figure 8: Bivalent β-carboline derivatives.

Figure 9: N9-heterodimeric β-carbolines as cytotoxic agents.
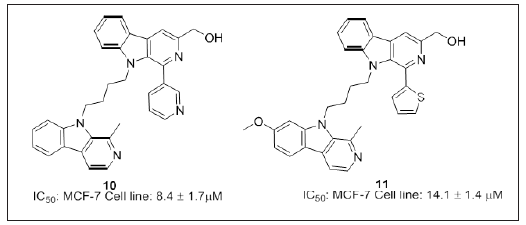
Figure 10: β-carboline conjugates as DNA intercalative
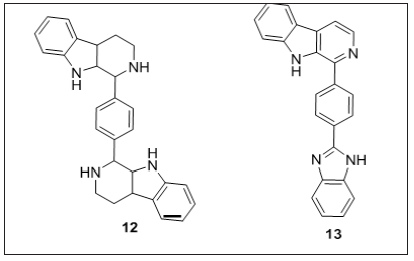
Shastri et al. [99] (Figure 10) reported compound 12 and 13 a series of β-carbolines with other heterocycles linked by phenyl ring with an anticancer activity (GI50 values range from 1.00 to 7.10μM) against all the cell lines. CT-DNA intercalation and protein binding studies showed that molecules are highly potent. Authors also demonstrated binding of compound 12 and 13 to DNA by docking studies. Compound 12 and 13 showed hydrogen bonding interactions with oxygen atom of carbonyl group of MET547 and hydrogen of amine group makes a hydrogen bonding interaction with LYS524. Both the compounds are surrounded by hydrophobic interactions (LEU528, LEU531, ALA527, GLY401, LEU505, PHE506, PHE508, LEU543, MET547, LEU582, ALA583, VAL575, and GLY571) and hydrophilic interactions (GLU503, ASN549, GLU548, THR578, SER577, THR507, LYS524, THR526, TYR400, GLN525, and LYS523). Hydrophobic and hydrophilic interactions play a major role in binding.
Later the same research group has explored potency of (+) and (−)-kumudine A (Figure 11), kumudine B-D and kumudine E against Hep3B and HepG2 cells by SRB assay. 14a ((+)- Kumudine B) and 14b ((-)-Kumudine B) were most potent and selective towards Hep3B cells whereas 14b showed superior cytotoxicity compared to 14a at same concentration. Thus, 14b may be a lead candidate for the development of anti-hepatoma agents [100].
Figure 11: (+)-Kumudine.

Cinnamide-β-carboline derivatives
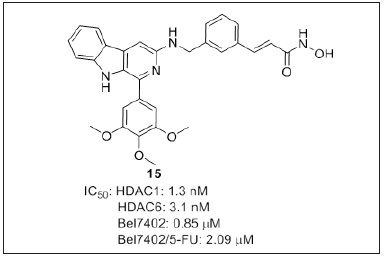
Ling et al. [101] investigated β-carboline based N-hydroxy cinnamide derivatives (Figure 12) for histone deacetylase inhibitory effect. Authors demonstrated that, the HDAC1 inhibitory activity of the synthesized compounds clearly depends on the substitution at C1 position of β-carboline. Aryl group substitution at C1 position of β-carboliine highly influences the inhibitory activity, where electron donating groups like mono-methoxyl or di/tri-methoxyl groups are more favorable compared to the electron withdrawing groups. Compound 15 was the most potent analogue with an IC50 value 0.85, 2.09μM against drug-sensitive Bel7402, drug-resistant Bel7402/5-FU cell lines and 1.3nM against HDAC1 were 5 to 6 fold better than SAHA (IC50 = 4.72-9.83μM) and 18-30 fold more potent than 5-FU (IC50 = 15.6-61.7μM). Compound 15 induce apoptosis by enhancing the expression of cleaved caspase-3 and PARP. 15 upregulate the LC3-II and down regulate the P62 and LC3-I. Thus, the author discloses β-carboline/N-hydroxycinnamamide hybrids as potential leads for the treatment of drug-resistant hepatocellular carcinoma.
Figure 12: Cinnamide linked β-carboline derivatives as HDAC inhibitors.
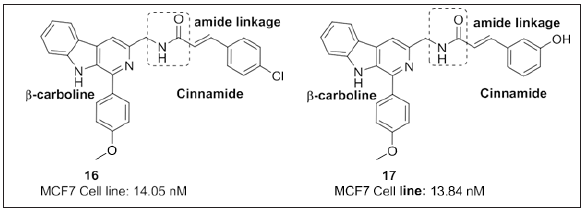
Kamal et al. [102] disclosed C3-trans-cinnamide linked β-carboline motifs and evaluated its cytotoxic potential (Figure 13). Authors states that, 4-methoxyphenyl group at position-1 and 3,4,5- trimethoxy on cinnamide part at position-3 were most active when compared to other conjugates and are crucial for in vitro cytotoxic activity whereas acrylamide containing congeners were less potent. 16 and 17 were potent against MCF-7 with an IC50 value 14.05nM and 13.84nM and catalytically inhibit topo-I. 16 and 17 are considered as potential candidates for anticancer therapy.
Figure 13: C3-trans-cinnamide based β-carbolines as Topo-I inhibitors.
Bisindole-β-carboline derivative
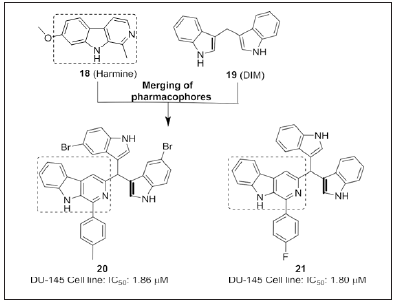
Kamal et al. [103] disclosed β-carboline linked bisindole congeners (Figure 14) for topo I inhibitory activity. SAR analysis revealed that substitutions like fluoro and methyl on the phenyl ring at C- 1 position displayed potent cytotoxicity. Replacement of methyl by methoxy displayed 1.4-fold decreased in the activity. Therefore, electron deficient substituents enhanced the cytotoxicity compared to electron rich substituents. Electron deficient substituents at C-5 position on indole ring enhances the activity compared to electron rich substituents. Compounds 20 and 21 inhibited the topoisomerase I at 20μM concentration. Therefore, the authors disclosed a potential scaffold 20 and 21 having combilexin type of interactions with DNA [103].
Figure 14: Lead optimization of β-carboline linked bisindole derivatives.
Coumarin-β-carboline derivative
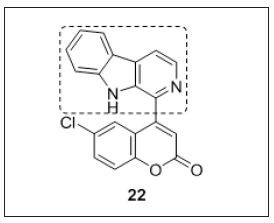
Amalgamation of tetrahydro-β-carboline (KSP protein inhibitor and antimitotic agent) and coumarin (tubulin inhibitor) may lead to the development of coumarin-β-carboline hybrids. Compound 22 showed good cytotoxic results compared to tetrahydro-β-carboline. Compound 22 (Figure 15) cleaves the CT-DNA in a conc. dependent manner. Molecular docking results revealed that coumarin ring in the compound 22 interacted with tubulin rather than β-carboline. Additionally, the authors docked compound 22 with KSP (Kinesin spindle protein), it shows interactions with β-carboline and there is no interaction with the coumarin. Therefore, these results revealed that structural modifications of compound 22 could be further explored for enhancing the selectivity and cytotoxicity [104].
Figure 15: Amalgamation of THBC linked coumarin.
Furan –β-carboline derivative
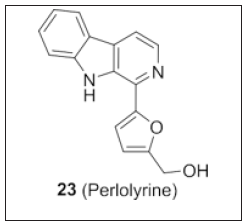
By using chromatographic separation techniques, isolated crinane type alkaloids from the leaves of Crinum latifolium and evaluated its cytotoxic potential on human cancer cell lines. Among the tested compounds, perlolyrine (Figure 16) showed potent cytotoxicity. Thus, the author discloses, perlolyrine as a lead candidate for anti-tumor property [105].
Figure 16: Perlolyrine.
Pharmacological importance of Eudistomin U
DNA binding studies of natural β-carboline alkaloid eudistomin U was examined by Mulcahy et al. [106] Further mechanistic studies were carried out and states that eudistomin U binds weakly when compared to other alkaloids. Thus, eudistomin U (Figure 17) can be a promising lead for the development of newer cytotoxic agents [106].
Figure 17: Eudistomin U.
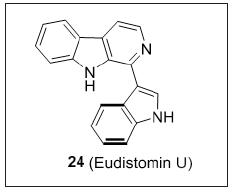
Salicylic acid-β-carboline derivative
Xu et al. [14] synthesized novel hybrids of β-carboline and salicylic acid (Figure 18). SAR studies revealed that methyl group at position-1 of the β-carboline unveiled strong anticancer activity than with hydrogen or p-methoxyphenyl. Length of the linker can influence the cytotoxic activity. Hybrids linked with butanediamine (n = 3) and amyl diamine (n = 4) exhibited greater potency than hexanediamine (n = 5). Most of the compounds in the series showed profound cytotoxicity than the standards 5-Fluorouracil and Harmine. Compound 27 selectively suppress the liver cancer cells (SMMC-7721). Mechanistic studies have shown that they decrease the mitochondrial membrane potential which was associated with the down regulation of Bcl-2 and upregulation of Bax in dose dependent manner. 27 can be considered as a novel molecule for the intervention of various cancers.
Figure 18: β-carboline linked salicylic acid derivatives.
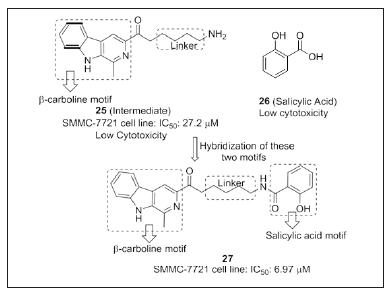
ydantoin, thiohydantoin and urea-THBC derivative
By employing structural diversity-oriented synthesis, Wang et al. [107] designed and synthesized a series of tetrahydro-β- carboline ester linked with hydantoin, thiohydantoin and urea motifs. Compounds 29, 30 and 31 (Figure 19) exhibited higher anti-TMV activity in vitro and in vivo than that of commercial plant virucide ribavirin. Some of the compounds showed good fungicidal and insecticidal activity against Plutella xylostella and Culex pipiens pallens. Hydantoin, thiohydantoin and urea motifs of these hybrids can improve the activities of the natural products. SAR studies states that substituents on thiohydantoin moiety have a great influence on anti-TMV activity. Sterically hindered substituents (R = isopropyl ≈ cyclohexyl > cyclopentyl > n-butyl) on thiohydantoin possesss better anti-TMV activity. Anti-TMV activity of the compound was increased if we change the substituent from phenyl to benzyl. In case of N-phenyl hydantoin, the compounds substituted with electron withdrawing groups shows profound activity compared to electron donating groups. The order of reactivity of substituents on ureas was isopropyl > cyclopentyl > t-butyl ≈ cyclohexyl. Hydantoin and urea compounds exhibit higher insecticidal property rather than thiohydantoin. Whereas, tetrahydro-β-carboline ester linked with hydantoin, thiohydantoin and urea derivatives are the potent scaffolds for possessing anti-TMV activity rather than the standard (ribavirin).
Figure 19: Lead optimization of THBC linked thiohydantoin, hydantoin and urea derivatives.
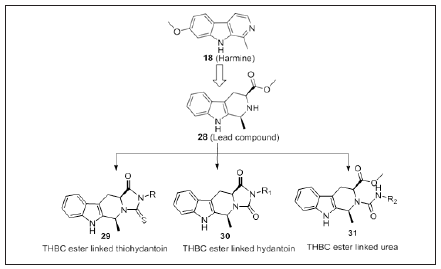
Hydroxamate-β-carboline derivatives
β-carboline based hydroxamate hybrids comprised of β-carboline as cap, benzylic as linker and hydroxamate as ZBG were tested against various cancer cell lines. SAR studies states that C1 substitution had significant effect on HDAC1 inhibitory activity. Compounds with electron rich groups (methoxy, methyl) at C1 position was more potent compared to electron deficient groups such as nitro. Compound 34 showed most potent activity with an IC50 value 0.53-1.56μM than standard drug Harmine (IC50: 46.7- 55.3 μM). Potency of 34 (Figure 20) against HepG2 cells was 15 and 16-fold lower than 33 and 32 (Figure 20). Further mechanistic studies revealed that 34 inhibit histone H3 and α-tubulin acetylation in dose dependent manner. Moreover, it arrests G2/M phase in HepG2 cells through inhibiting the cell cycle related protein CDK1 and cyclin B in dose dependent manner. 34 reduced the protein level of MMP2 and MMP9 thereby inhibit MAPK pathway. Thus 34 can be considered as a potential candidate for the development of antitumor agents in case of Hepatocellular carcinoma [108].
Figure 20: β-carboline hydroxamates.
Phenylalanine-β-carboline derivative
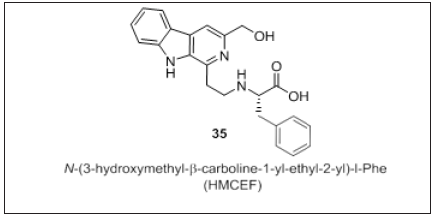
Wu et al. [109] developed a P-selectin inhibitor (Figure 21) capable of inhibiting thrombosis and inflammation. HMCEF is a nanoscaled antitumor drug, forms nanoparticles with a diameter of <120nm that promote delivery in blood circulation. HMCEF intercalates with DNA and inhibit the proliferation of cells. Thus, the author discloses HMCEF is a promising antitumor drug used in thrombosis and inflammation patients.
Figure 21: HMCEF as P-selectin inhibitor.
Imidazolium –THBC derivative
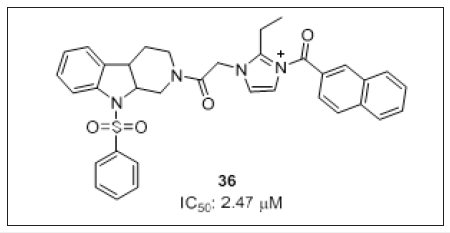
To design MEK-1 inhibitors, Meng et al. [110] employed in silico approaches for the construction of N-substituted tetrahydro-β- carboline imidazolium salt (Figure 22) derivatives and its potential target was identified by QASR, PharmMapper and molecular docking studies. Molecular docking studies demonstrated that target protein was stable for 0.8–5ns. Benzenesulfonylated substitution in compound 36 showed ligand receptor interaction with Lys192, naphthyl ring showed aromatic interactions with Asp208 or Phe209. Thus, suggesting N-substituted tetrahydro- β-carboline and imidazole as a promising scaffold for the development of MEK- 1 inhibitors.
Figure 22: Tetrhydro-β-carboline imidazolium salt as MEK-1 inhibitor.
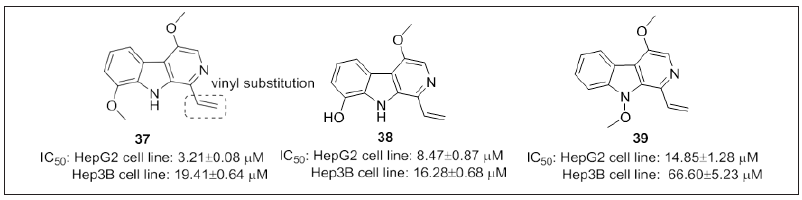
Huang et al. [111] identified 39 β-carboline alkaloids from picrasma quassioides. Compound 37, 38 and 39 (Figure 23) were the most potent compounds comparable with sorafinib (IC50: 8.35μM) and shows better activity than 5-FU (IC50: 27.06μM). SAR studies revealed that double bond at C-3 position enhances the activity and order of reactivity: vinyl > acetyl > aldehyde > ester group. Two oxygen substitutions in the structure displayed better activity. Potent compounds induce apoptosis via activating caspase 3. These hybrids represent valuable complement to existing chemotherapies.
Figure 23: β-carboline derivatives.
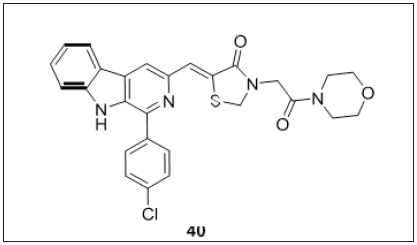
Thiazolidinedione-β-carboline derivative
Shankaraiah et al. [112] (Figure 24) designed a series of β-carboline-thiazolidinedione hybrids and tested against various cancer cell lines. SAR analysis clearly indicated that C1 position of β-carboline bearing benzaldehyde substituted with electron withdrawing group at para position displayed better cytotoxic activity rather than electron donating groups. Compound 40 was the most potent against MDA-MB-231 with an IC50 value 0.97±0.13μM. Further pharmacological studies states that compound 40 arrest the cell cycle at subG1 phase. Spectroscopic and molecular modelling studies showed the classical interaction with CT-DNA bearing the binding constant value 1×105 M-1.
Figure 24: β-carboline based thiazolidinedione derivatives.
β-carbolinium bromide derivatives
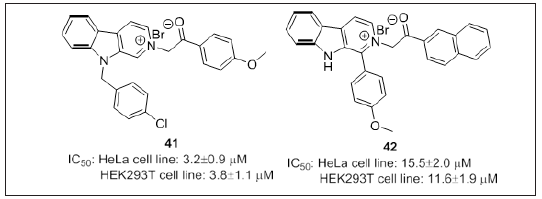
Dalip kumar et al. [113] synthesized β-carbolinium bromides (Figure 25) from easily available starting materials i.e., β-carbolines and 1-aryl-2-bromoethanones. Most potent derivative 41 tested against BxPC-3, HeLa, C4-2, PC-3, HEK293T and MDA-MB- 231cancer cell line with an IC50 value 3.16-7.93μM. In order to understand the in-depth mechanism of action, 41 and 42 were exposed to castration resistant prostate cancer cell line (C4–2) and resulted in increased levels of cleaved PARP1 as well as inhibited the tubulin polymerization. From the results, it can be observed that modifications in the structure of β-carbolinium bromides may ensue potent cytotoxic agents.
Figure 25: β-carbolinium bromides as tubulin inhibitors.
Porphyrin-β-carboline derivative
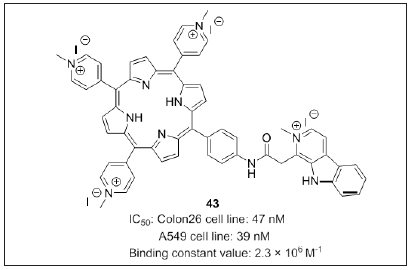
Dalip kumar et al. [114] developed a microwave assisted approach to prepare water-soluble cationic porphyrin-β-carboline conjugates (Figure 26) by coupling β-carboline acid and 5-(4- aminophenyl) tripyridyl porphyrin. N-Methylation of porphyrin-β- carboline conjugate rapidly afforded to form cationic porphyrin-β- carboline. Compound 43 was the most potent against colon26 and A549 cell line with an IC50 value: 47nM and 39nM. Additionally, porphyrin-β- carboline conjugate 43 possess binding constant (Kb) value 2.3×106 M-1 similar to H2TMPyP (2.5×106 M-1) displayed visible light induced DNA cleavage and triggered efficient cell death. Thus compound 43 was proved to be a novel and potent photosensitizing agent and likely to be a potential candidate for PDT.
Figure 26: Porphyrin linked β-carbolines.
Trifluoromethylated –THBC derivative
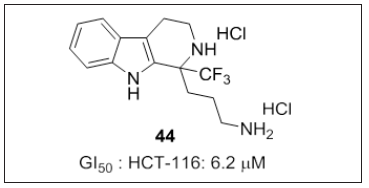
Kakali Bhadra et al. [16] reported a series of trifluoromethylated carboline (Figure 27) compounds with an additional amino alkyl (α- or δ-position) and guanidine (α-position) alkyl chains of varying length. SAR analysis revealed that incorporation of trifluoromethyl group could significantly improve the metabolic stability, lipophilicity, and other physicochemical properties of target molecules. Binding affinity with CT-DNA decreases with increase chain length because of its bulky nature. Order of reactivity towards DNA binding: γ-carboline > β with amino alkyl chain > guanidine alkyl chain. Compound 44 showed potent cytotoxicity with GI50 6.2μM against HCT- 116 cell line. β-carboline with amino alkyl chain possess poor cytotoxicity. Mode of binding and partial interaction was supported by viscosity studies and FTIR. These results may be useful for designing novel carboline derivatives for improved therapeutic applications in future.
Figure 27: Trifluoromethylated carboline derivatives.
Indolinone-β-carboline derivative
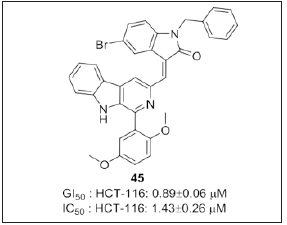
Shankaraiah et al. [115] synthesized a series of (E)- 3((1aryl 9Hpyrido[3,4b] indol 3yl) methylene indolin-2-one congeners (Figure 28) and evaluated for their in vitro cytotoxic activity. Compound 45 showed potent cytotoxicity with an IC50 of 1.43±0.26μM and GI50 value of 0.89±0.06μM respectively. Further, mechanistic studies were performed by using various assays such as annexin V-FITC/PI, DCFDA, and JC-1to understand the in-depth mechanism of action. Compound 45 arrested the cell cycle at G0/G1 phase. Additionally, western blot analysis indicated that compound 45 on HCT-15 cancer cells led to decreased expression of Bcl-2 and increased protein expression of pro-apoptotic proteins such as Bax, caspase-3, 8, 9 and cleaved PARP with reference to actin.
Figure 28: β-carboline linked indolinone conjugates.
Indole-β-carboline derivative
Ke et al. [116] synthesized a series of β-carboline amide derivatives (Figure 29) from natural marine alkaloid Pityriacitrin and evaluated there in vitro cytotoxic potential. Compound 46 with sulfonyl group possess highest inhibitory activity against SGC-7901 (IC50: 6.82±0.98μM), A875 (IC50: 8.43±1.93μM), HepG2 (IC50: 7.69±2.17μM), MARC145 (IC50: 7.19±1.43μM) respectively. The author discloses compound 46 might be a lead molecule for development of novel cytotoxic agents.
Figure 29: β-carboline amides as cytotoxic agents.

Podophyllotoxin-β-carboline derivative
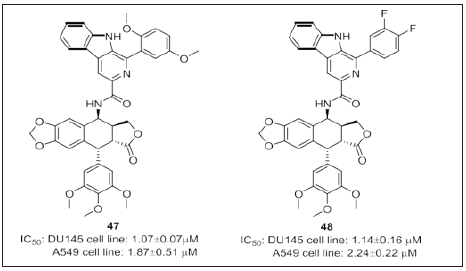
By utilizing the molecular hybridization strategy, Kamal et al. [117] (Figure 30) has synthesized a series of podophyllotoxin linked β-carboline conjugates and evaluated their cytotoxic potential and Topo II inhibitory activity. 47 and 48 were the most potent among the series of compounds. External binding affinity of compounds 47 and 48 was disclosed by DNA binding studies. Detailed biological studies such as cell cycle analysis, Comet assay, DNA binding studies and topoisomerase II inhibition studies have revealed that these congeners are DNA interacting topoisomerase II inhibitors. Molecular docking studies states that all the interactions strengthen through minor groove binding affinity.
Figure 30: Podophyllotoxin based β-carbolines as Topo-II inhibitors.
Conclusion and Outlook
Natural products act as a creative source for drug-leads and are deep-seated in drug discovery due to their biological activity and wider chemical space. Synthetic renewal of these natural products to semi-synthetic derivatives and natural product like molecules with clinical significance is an attractive area for chemists. β-carbolines represent an importance class of indolebased alkaloids with wide spectrum of anticancer activities exerting through varied mechanisms by interacting with different enzymes/targets/receptors. The prerequisite for the development of novel β-carbolines as a potential anticancer agent include site specificity, enhancing potency with improved pharmacokinetic profile, metabolic stability with minimal side effects, improvement in the bioavailability and incidence of drug resistance. In this review, attempts have been taken to focus the occurrence, structural diversity, highlighted the latest information available on anticancer attributes of β-carbolines with the addition of relevant SAR and binding interactions studied through molecular docking, mainly covering the years 2017-19. Further, we believe that variedly functionalized β-carboline derivatives and β-carboline hybrids integrated in this review will help to improve the status of this privileged scaffold in its future synthesis for drug discovery applications.
Conflict of interest
The authors confirm that this article content has no conflict of interest.
Acknowledgement
The authors thank the Department of Pharmaceuticals, Ministry of Chemicals and Fertilizers Govt. of India, New Delhi for the award of NIPER fellowship.
References
- Newman DJ, Cragg GM (2010) Natural products as sources of new drugs over the 30 years from 1981 to 2010. J Nat Prod 75(3): 311-335.
- Brahmachari G (2011) Natural products in drug discovery: Impacts and opportunities-an assessment. Bioactive Natural Products, pp. 1-199.
- Chen Y, Garcia de Lomana M, Friedrich NO, Kirchmair J (2018) Characterization of the chemical space of known and readily obtainable natural products. J Chem Inf Model 58(8): 1518-1532.
- Butler MS (2004) The role of natural product chemistry in drug discovery. J Nat Prod 67(12): 2141-2153.
- Siddiqui AA, Iram F, Siddiqui S, Sahu K (2014) Role of natural products in drug discovery process. Int J Drug Dev & Res 6(2): 172-204.
- Cragg GM, Grothaus PG, Newman DJ (2009) Impact of natural products on developing new anti-cancer agents. Chem Rev 109(7): 3012-3043.
- Koehn FE, Carter GT (2005) The evolving role of natural products in drug discovery. Nat Rev Drug Discov 4(3): 206-220.
- De Corte BL (2016) Underexplored opportunities for natural products in drug discovery. J Med Chem 59(20): 9295-9304.
- Lee KH (2010) Discovery and development of natural product-derived chemotherapeutic agents based on a medicinal chemistry approach. J Nat Prod 73(3): 500-516.
- Newman DJ (2008) Natural products as leads to potential drugs: An old process or the new hope for drug discovery? J Med Chem 51(9): 2589-2599.
- Vasilevich NI, Kombarov RV, Genis DV, Kirpichenok MA (2012) Lessons from natural products chemistry can offer novel approaches for synthetic chemistry in drug discovery. J Med Chem 55(16): 7003-7009.
- Ertl P, Roggo S, Schuffenhauer A (2008) Natural product-likeness score and its application for prioritization of compound libraries. J Chem Inf Model 48(1): 68-74.
- Walters WP, Green J, Weiss JR, Murcko MA (2011) What do medicinal chemists actually make? A 50-year retrospective. J Med Chem 54(19): 6405-6416.
- Xu QB, Chen XF, Feng J, Miao JF, Liu J, et al. (2016) Design, synthesis and biological evaluation of hybrids of β-carboline and salicylic acid as potential anticancer and apoptosis inducing agents. Scientific Reports 6: 36238.
- Smirnova OB, Golovko TV, Granik VG (2011) Carbolines. part 2: Comparison of some of the properties of α-, γ- and δ-carbolines (Review). Pharmaceut Chem J 45(7): 389-400.
- Sarkar S, Shmatova OI, Nenajdenko VG, Bhadra K (2019) Trifluoromethylated carboline compounds targeting DNA: Synthesis, binding and anti-proliferative effects on human cancer cell lines. Bioorg Chem 86: 61-79.
- Cao R, Peng W, Wang Z, Xu A (2007) beta- carboline alkaloids: Biochemical and pharmacological functions. Curr Med Chem 14(4): 479-500.
- Huang H, Yao Y, He Z, Yang T, Ma J, et al. (2011) Antimalarial β-carboline and indolactam alkaloids from marinactinospora thermotolerans, a deep-sea isolate. J Nat Prod 74(10): 2122-2127.
- Li Y, Yan W, Yang J, Yang Z, Hu M, et al. (2018) Discovery of novel β- carboline/acylhydrazone hybrids as potent antitumor agents and overcome drug resistance. Eur J Med Chem 152: 516-526.
- Gu H, Li N, Dai J, Xi Y, Wang S (2018) Synthesis and in vitro antitumor activity of novel bivalent β-carboline-3-carboxylic acid derivatives with dna as a potential target. Int J Mol Sci 19(10): 3179.
- Louis ED, Zheng W (2010) β-Carboline alkaloids and essential tremor: exploring the environmental determinants of one of the most prevalent neurological diseases. The Scientific World Journal 10: 1783-1794.
- Polanski W, Reichmann H, Gille G (2011) Stimulation, protection and regeneration of dopaminergic neurons by 9-methyl-β-carboline: A new anti-Parkinson drug? Expert Rev Neurother 11(6): 845-860.
- Rook Y, Schmidtke KU, Gaube F, chepmann D, Wunsch B, et al. (2010) Bivalent β-carbolines as potential multitarget anti-Alzheimer agents. J Med Chem 53(9): 3611-3617.
- Volk RB, Furkert FH (2006) Antialgal, antibacterial and antifungal activity of two metabolites produced and excreted by cyanobacteria during growth. Microbiological Res 161(2): 180-186.
- Shin HJ, Lee HS, Lee DS (2010) The synergistic antibacterial activity of 1-acetyl-beta-carboline and beta-lactams against methicillin-resistant Staphylococcus aureus (MRSA). J MicrobiolBiotechnol 20(3): 501- 505.
- Rao KV, Kasanah N, Wahyuono S, Tekwani BL, Schinazi RF, et al. (2004) Three new manzamine alkaloids from a common Indonesian sponge and their activity against infectious and tropical parasitic diseases. J Nat Prod 67(8): 1314-1318.
- Rinehart Jr KL, Kobayashi J, Harbour GC, Gilmore J, Mascal M, et al. (1987) Eudistomins A-Q.beta.-carbolines from the antiviral Caribbean tunicate Eudistoma olivaceum. J Am Chem Soc 109(11): 3378-3387.
- Frederic, Bruyere C, Vancraeynest C, Reniers J, Meinguet C, et al. (2012) Novel trisubstituted harmine derivatives with original in vitro anticancer activity. J Med Chem 55(14): 6489-6501.
- Li Y, Liang F, Jiang W, Yu F, Cao, et al. (2007) DH334, a β-carboline anti-cancer drug, inhibits the CDK activity of budding yeast. Cancer Biol Ther 6(8): 1204-1210.
- Barsanti PA, Wang W, Ni ZJ, Duhl D, Brammeier N, et al. (2010) The discovery of tetrahydro-β-carbolines as inhibitors of the kinesin Eg5. Bioorg Med Chem Lett 20(1): 157-160.
- Kamal A, Srinivasulu V, Nayak VL, Sathish M, Shankaraiah N, et al. (2014) Design and synthesis of C3‐pyrazole/chalcone‐Linked beta‐carboline hybrids: Antitopoisomerase I, DNA‐interactive, and apoptosis‐inducing anticancer agents. Chem Med Chem 9(9): 2084-2098.
- Jimenez J, Riveron-Negrete L, Abdullaev F, Espinosa-Aguirre J, Rodríguez-Arnaiz (2008) Cytotoxicity of the β-carboline alkaloids harmine and harmaline in human cell assays in vitro. Exp Toxicol Pathol 60(4- 5): 381-389.
- Trujillo JI, Meyers MJ, Anderson DR, Hegde S, Mahoney MW, et al. (2007) Novel tetrahydro-β-carboline-1-carboxylic acids as inhibitors of mitogen activated protein kinase-activated protein kinase 2 (MK-2). Bioorg Med Chem Lett 17(16): 4657-4663.
- Zhang J, Li Y, Guo L, Cao R, Zhao P, et al. (2009) DH166, a beta-carboline derivative, inhibits the kinase activity of PLK1. Cancer Biol Ther 8(24): 2374-2383.
- Li X, Bai B, Liu L, Ma P, Kong L, et al. (2015) Novel β-carbolines against colorectal cancer cell growth via inhibition of Wnt/β-catenin signaling. Cell Death Dis 1(1): 1-9.
- Göckler N, Jofre G, Papadopoulos C, Soppa U, Tejedor FJ, et al. (2009) Harmine specifically inhibits protein kinase DYRK1A and interferes with neurite formation. The FEBS Journal 276(21): 6324-6337.
- Seifert A, Allan LA, Clarke PR (2008) DYRK1A phosphorylates caspase 9 at an inhibitory site and is potently inhibited in human cells by harmine. The FEBS Journal 275(24): 6268-6280.
- Wu J, Zhao M, QianK, Lee KH, Morris-Natschke S, et al.(2009) Novel N-(3-carboxyl-9- benzyl-β-carboline-1-yl) ethylamino acids: Synthesis, anti-tumor evaluation, intercalating determination, 3D QSAR analysis and docking investigation. Eur J Med Chem 44(10): 4153-4161.
- Hagen TJ, Skolnick P, Cook JM (1987) Synthesis of 6-substituted beta-carbolines that behave as benzodiazepine receptor antagonists or inverse agonists. J Med Chem 30(4): 750-753.
- Manasa KL, Tangella Y, Ramu G, Babu BN (2017) TCCA; A mild reagent for decarboxylative/dehydrogenative aromatization of tetrahydro-β-carbolines: Utility in the total synthesis of norharmane, harmane, eudistomin U, I and N. Chemistry Select 2(28): 9162-9167.
- Grella B, Teitler M, Smith C, Herrick-Davis K, Glennon RA (2003) Binding of β- carbolines at 5-HT2 serotonin receptors. Bioorg Med Chem Lett 13(24): 4421-4425.
- Zheng H, Li L, Sun B, Gao Y, Song W, et al. (2018) Design and synthesis of furyl/thienyl pyrroloquinolones based on natural alkaloid perlolyrine, lead to the discovery of potent and selective PDE5 inhibitors. Eur J Med Chem 150: 30-38.
- Hayashi K, Nagao M, Sugimura T (1977) Interactions of norharman and harman with DNA. Nucleic Acids Res 4(11): 3679-3686.
- Madle E, Obe G, Hansen J, Ristow H (1981) Harman and norharman: Induction of sister- chromatid exchanges in human peripheral lymphocytes in vitro and interaction with isolated DNA. Mutat Res Genet Toxicol 90(4): 433-442.
- Mita S, Kamataki T, Kato R (1984) Effect of norharman on DNA strand breaks and mutation of Chinese hamster V79 cells by chemical carcinogens. Carcinogenesis. 5(6): 715-718.
- Shimoi K, Kawabata H, Tomita I (1992) Enhancing effect of heterocyclic amines and ß- carbolines on UV or chemically induced mutagenesis in coli. Mutat Res Fund Mol M 268(2): 287-295.
- Funayama Y, Nishio K, Wa abayashi K, Nagao M, Himoi K, e t al. (1996) Effects of β- and γ-carboline derivatives on DNA topoisomerase activities. Mutat Res Fund Mol M 349(2); 183-191.
- Herraiz T, Chaparro C (2006) Human monoamine oxidase enzyme inhibition by coffee and β-carbolines norharman and harman isolated from coffee. Life Sci 78(8): 795-802.
- Kühn-Velten WN (1993) Norharman (β-carboline) as a potent inhibitory ligand for steroidogenic cytochromes P450 (CYP11 and CYP17). Eur J Pharmacol 250(1): R1-R3.
- Okereke CS (2002) Role of integrative pharmacokinetic and pharmacodynamic optimization strategy in the management of Parkinson’s disease patients experiencing motor fluctuations with levodopa. J Pharm Sci 5(2): 146-161.
- Kim H, Sablin O, Ramsay (1997) Inhibition of monoamine oxidase A by β- carboline derivatives. Arch Biochem Biophys 337(1): 137-142.
- Warner HR, Persson ML, Bensen RJ, Mosbaugh DW, Linn S (1981) Selective inhibition by harmane of the apurinic/apyrimidinic endonuclease activity of phage T4-induced UV endonuclease. Nucleic Acids Res 9(22): 6083-6092.
- Ishida J, Wang HK, Oyama M, Cosentino ML, Hu, CQ, et al. (2001) Anti-AIDS agents. Anti-HIV activity of harman, an anti-HIV principle from Symplocos setchuensis, and its derivatives. J Nat Prod 64(7): 958-960.
- Di Giorgio C, Delmas F, Ollivier E, Elias R, Balansard G, et al. (2004) In vitro activity of the β-carboline alkaloids harmane, harmine, and harmaline toward parasites of the species Leishmania infantum. Exp Parasitol 106(3-4): 67-74.
- Cao R, Peng W, Chen H, Ma Y, Liu X (2005) DNA binding properties of 9-substituted harmine derivatives. Biochem Biophys Res Commun 338(3): 1557-1563.
- Shimoi K, Kawabata H, Tomita I (1992) Enhancing effect of heterocyclic amines and ß- carbolines on UV or chemically induced mutagenesis in coli. Mutat Res Fund Mol M 268(2): 287-295.
- Song Y, Wang J, Teng F, Kesuma D, Deng Y, et al. (2002) β-Carbolines as specific inhibitors of cyclin-dependent kinases. Bioorg Med Chem Lett 12(7): 1129-1132.
- Hudson JB, Graham EA, Fong R, Hudson LL, Towers GHN (1986) Further studies on the antiviral activity of harmine, a photoactive β‐carboline alkaloid. Photochem Photobiol 44(4): 483-487.
- Abe A, Yamada H, Moriya, Miyazawa K (2011) The β-carboline alkaloid harmol induces cell death via autophagy but not apoptosis in human non-small cell lung cancer A549 cells. Biol Pharm Bull 34(8): 1264-1272.
- Dejos C, Voisin P, Bernard M, Gnacq M, Berges T (2014) Canthin-6-one displays antiproliferative activity and causes accumulation of cancer cells in the G2/M phase. J Nat Prod 77(11): 2481-2487.
- Xu Z, Chang FR, Wang HK, Kashiwada Y, McPhail AT, et al. (2000) Anti-HIV Agents 45 and Antitumor Agents 205. Two New Sesquiterpenes, Leitneridanins A and B, and the Cytotoxic and Anti-HIV Principles from Leitneria floridana. J Nat Prod 2000 63(12): 1712-1715.
- Kolodina AA, Serdyuk OV (2018) Eudistomin U, Isoeudistomin U, and related indole compounds: Synthesis and biological activity. Heterocycles 96(7): 1171-1196.
- Youssef DT (2005) Hyrtioerectines A- C, Cytotoxic Al aloids from the ed ea ponge Hyrtios e rectus. J Nat Prod 68(9): 1416-1419.
- Sandler J, Colin PL, Hooper JN, Faulkner DJ (2002) Cytotoxic β-Carbolines and Cyclic Peroxides from the palauan sponge Plakortis nigra. J Nat Prod 65(9): 1258-1261.
- Ashok P, Ganguly S, Murugesan S (2014) Manzamine alkaloids: isolation, cytotoxicity, antimalarial activity and SAR studies. Drug Discovery Today 19(11): 1781-1791.
- Bharate S, Manda S, Mupparapu N, Bathini N, Vishwakarma R (2012) Chemistry and biology of fascaplysin, a potent marine-derived CDK-4 inhibitor. Mini Rev Med Chem 12(7): 650-664.
- Soni R, Muller L, Furet P, Schoepfer J, Stephan C, et al. (2000) Inhibition of cyclin- dependent kinase 4 (Cdk4) by fascaplysin, a marine natural product. Biochem Biophys Res Comm 275(3): 877-884.
- Shimoi K, Kawabata H, Tomita I (1992) Enhancing effect of heterocyclic amines and ß- carbolines on UV or chemically induced mutagenesis in coli. Mutat Res Fund Mol M 268(2): 287-295.
- Kamins y M, Levy O, Garry MT, Carrasco N (1991) Inhibition of the Na+/I‐symporter by harmaline and 3‐amino‐1‐methyl‐5H‐pyrido (4, 3‐b) indole acetate in thyroid cells and membrane vesicles. Eur J Biochem 200(1): 203-207.
- Di Giorgio C, Delmas F, Ollivier E, Elias R, Balansard G, et al. (2004) In vitro activity of the β-carboline alkaloids harmane, harmine, and harmaline toward parasites of the species Leishmania infantum. Exp Parasitol 106(3-4): 67-74.
- El Gendy MA, Soshilov AA, Denison MS, El-Kadi AO (2012) Harmaline and harmalol inhibit the carcinogen-activating enzyme CYP1A1 via transcriptional and posttranslational mechanisms. Food Chem Toxicol 50(2): 353-362.
- Wang KB, Yuan CM, Xue CM, Li DH, Jing YK, et al. (2014) Pegaharmalines A and B, two novel β-carboline alkaloids with unprecedented carbon skeletons from Peganum harmala. RSC Advances 4(96): 53725-53729.
- Wang KB, Li DH, Hu P, Wang WJ, Lin C, et al. (2016) A series of β-carboline alkaloids from the seeds of Peganum harmala show G-quadruplex interactions. Org Lett 18(14): 3398-3401.
- Wang KB, Di YT, Bao Y, Yuan CM, Chen G, et al. (2014) Peganumine A, a β-carboline dimer with a new octacyclic scaffold from Peganum harmala. Org Lett 16(15): 4028-4031.
- Soares PR, De Oliveira PL, De Oliveira CM, Kato L, Guillo LA (2012) In vitro antiproliferative effects of the indole alkaloid vallesiachotamine on human melanoma cells. Arch Pharmacal Res 35(3): 565-571.
- Liu BPL, Chong EYY, Cheung FWK, Duan JA, Che CT, et al. (2005) Tangutorine induces p21 expression and abnormal mitosis in human colon cancer HT-29 cells. Biochem Pharmacol 70(2): 287-299.
- Samita F, Ochieng CO, Owuor PO, Manguro LOA, Midiwo JO (2017) Isolation of a new β-carboline alkaloid from aerial parts of Triclisia sacleuxii and its antibacterial and cytotoxicity effects. Nat Prod Res 31(5): 529-536.
- Lake RJ, Blunt JW, Munro MHG (1989) Eudistomins from the New Zealand ascidian Ritterella sigillinoides. Aust J Chem 42(7): 1201-1206.
- Gul W, Hamann MT (2005) Indole alkaloid marine natural products: An established source of cancer drug leads with considerable promise for the control of parasitic, neurological and other diseases. Life Sci 78(5): 442-453.
- Ota Y, Chinen T, Yoshida K, Kudo S, Nagumo Y, et al. (2016) Eudistomin C, an antitumor and antiviral natural product, targets 40S ribosome and inhibits protein translation. Chem Bio Chem 17(17): 1616-1620.
- Yamanokuchi R, Imada K, Miyazaki M, Kato H, Watanabe T, et al. (2012) Hyrtioreticulins A–E, indole alkaloids inhibiting the ubiquitin-activating enzyme, from the marine sponge Hyrtios reticulatus. Bioorg Med Chem 20(14): 4437-4442.
- Ashok P, Lathiya H, Murugesan S (2015) Manzamine alkaloids as antileishmanial agents: A review. Eur J Med Chem 97(5): 928-936.
- Ovenden SP, Nielson JL, Liptrot CH, Willis RH, Tapiolas DM, et al. (2011) Callophycin A, a cytotoxic tetrahydro-β-carboline from the red algae Callophycus oppositifolius. Phytochem Lett 2011 4(2): 69-71.
- Sonnenschein RN, Farias JJ, Tenney K, Mooberry SL, Lobkovsky E, et al. (2004) A further study of the cytotoxic constituents of a milnamide-producing sponge. Org Lett 6(5): 779-782.
- Foderaro TA, Barrows L, Lassota P, Ireland CM (1997) Bengacarboline, a New β- carboline from a marine Ascidian Didemnum J Org Chem 62(17): 6064-6065.
- Santos LS, Theoduloz C, Pilli RA, Rodriguez J (2009) Antiproliferative activity of arborescidine alkaloids and derivatives. EurJ Med Chem 44(9): 3810-3815.
- Williams DE, Davies J, Patrick BO, Bottriell H, Tarling T, et al. (2008) Cladoniamides A−G, tryptophan-derived alkaloids produced in culture by Streptomyces uncialis. Org Lett 10(16): 3501-3504.
- Takahashi Y, Kubota T, Fromont J, Kobayashi JI (2008) Zamamidines A and B, new manzamine alkaloids from the sponge Amphimedon Org Lett 11(1): 21-24.
- Stavrinides A, Tatsis EC, Foureau E, Caputi L, Kellner F, et al. (2015) Unlocking the diversity of alkaloids in Catharanthus roseus: Nuclear localization suggests metabolic channeling in secondary metabolism. Chem Biol 22(3): 336-341.
- Fischhof PK, Möslinger-Gehmayr, Herrmann WM, Friedmann A, uβmann DL (1996) Therapeutic efficacy of vincamine in dementia. Neuropsychobiology 34(1): 29-35.
- Rajapaksa NS, McGowan MA, Rienzo M, Jacobsen EN (2013) Enantioselective total synthesis of (+)-reserpine. Org Lett 15(3): 706-709.
- Abdelsalam MA, AboulWafa OM, Badawey ESA, El-Shoukrofy MS, El-Miligy MM, et al. (2018) Design and synthesis of some β-carboline derivatives as multi-target anticancer agents. Fut Med Chem 10(24): 2791-2814.
- Nekkanti S, Pooladanda V, Veldandi M, Tokala R, Shankaraiah N (2017) Synthesis of 1, 2, 3‐Triazolo‐fused‐tetrahydro‐β‐carboline derivatives via 1, 3‐dipolar cycloaddition reaction: Cytotoxicity evaluation and DNA‐binding studies. Chemistry Select 2(24); 7210-7221.
- Zhang ZJ, Zhang JJ, Jiang ZY, Zhong GH (2017) Design, synthesis, and bioactivity evaluation of novel β-carboline 1, 3, 4-oxadiazole derivatives. Molecules 22(11): 1811.
- Li Y, Yan W, Yang J, Yang Z, Chen L, et al. (2018) Discovery of novel β- carboline/acylhydrazone hybrids as potent antitumor agents and overcome drug resistance. Eur J Med Chem 152: 516-526.
- Qin JJ, Wang W, Li X, Deokar H, Zhang R, et al. (2018) Inhibiting β-catenin by β-carboline-type MDM2 Inhibitor for pancreatic cancer therapy. Front Pharmacol 9: 5.
- Jeyapal GP, Krishnasamy R, Suzuki CK, Venkatesh S, Chandrasekar MJN (2019) In- silico design and synthesis of N9-substituted β-Carbolines as PLK-1 inhibitors and their in-vitro/in-vivo tumor suppressing evaluation. Bioorg Chem 88: 102913.
- Guo L, Ma Q, Chen W, Fan W, Dai B (2019) Synthesis and biological evaluation of novel N 9-heterobivalent β-carbolines as angiogenesis inhibitors. J Enzyme Inhib Med Chem 34(1): 375-387.
- Samundeeswari S, Chougala B, Holiyachi M, Shastri L, Kulkarni M, et al. (2017) Design and synthesis of novel phenyl-1, 4-beta-carboline-hybrid molecules as potential anticancer agents. Eur J Med Chem 128: 123-139.
- Zhao WY, Zhou WY, Chen JJ, Yao GD, Lin B, et al. (2019) Enantiomeric β-carboline dimers from Picrasma quassioides and their anti-hepatoma potential. Phytochemistry 159: 39-45.
- Ling Y, Gao WJ, Ling C, Liu J, Meng C, et al. (2019) β-Carboline and N- hydroxycinnamamide hybrids as anticancer agents for drug-resistant hepatocellular carcinoma. Eur J Med Chem 168: 515-526.
- Sathish M, Dushantrao SC, Nekkanti S, Tokala R, Kamal A, et al. (2018) Synthesis of DNA interactive C3-trans-cinnamide linked β-carboline conjugates as potential cytotoxic and DNA topoisomerase I inhibitors. Bioorg Med Chem 26(17): 4916-4929.
- Kovvuri J, Nagaraju B, Nayak VL, Akunuri R, Kamal A, et al. (2018) Design, synthesis and biological evaluation of new β-carboline-bisindole compounds as DNA binding, photocleavage agents and topoisomerase I inhibitors. Eur J Med Chem 143: 1563-1577.
- Samundeeswari S, Kulkarni MV, Joshi SD, Dixit SR, Jayakumar S, et al. (2016) Synthesis and human anticancer cell line studies on coumarin‐β‐carboline hybrids as possible antimitotic agents. Chemistry Select 1(15): 5019-5024.
- Hanh TTH, Huong PTT, Van Thanh N, Trung NQ, Van Cuong T, et al. (2018) Crinane, augustamine, and β-carboline alkaloids from Crinum latifolium. Phytochem Lett 24: 27- 30.
- Giulietti JM, Tate PM, Cai A, Cho B, Mulcahy SP (2016) DNA-binding studies of the natural β-carboline eudistomin U. Bioorg Med Chem Lett 26(19): 4705-4708.
- Huang Y, Guo Z, Song H, Liu Y, Wang L, et al. (2018) Design, synthesis, and biological activity of β-carboline analogues containing hydantoin, thiohydantoin, and urea moieties. J Agric Food Chem 66(31): 8253-8261.
- Ling Y, GuoJ, Yang Q, Zhu P, Miao J, et al. (2018) Development of novel β-carboline- based hydroxamate derivatives as HDAC inhibitors with antiproliferative and antimetastatic activities in human cancer cells. Eur J Med Chem 144: 398-409.
- Wu J, Zhao M, Wang Y, Wang Y, Zhu H, et al. (2017) N-(3-hydroxymethyl-β- carboline-1-yl-ethyl-2-yl)-L-Phe: development toward a nanoscaled antitumor drug capable of treating complicated thrombosis and inflammation. Drug Des Devel Ther 11: 225-239.
- Liang J, Wang M, Li X, He X, Cao C, et al. (2017) Determination of structural requirements of N-substituted tetrahydro-β-carboline imidazolium salt derivatives using in silico approaches for designing MEK-1 inhibitors. Molecules 22(6): 1020.
- Zhao WY, Shang XY, Zhao L, Yao GD, Sun Z, et al. (2018) Bioactivity-guided isolation of β-Carboline alkaloids with potential anti-hepatoma effect from Picrasma quassioides (D. Don) Benn. Fitoterapia 130: 66-72.
- Tokala R, Thatikonda S, Sana S, Regur P, Godugu C, et al. (2018) Synthesis and in vitro cytotoxicity evaluation of β-carboline-linked 2, 4-thiazolidinedione hybrids: potential DNA intercalation and apoptosis-inducing studies. New J Chem 42(19): 16226- 16236.
- Reddy PV, Mishra S, Tantak MP, Nikhil K, Dalip K, et al. (2017) Design, synthesis, and in vitro cytotoxicity studies of novel β-carbolinium bromides. Bioorg Med Chem Lett 27(6): 1379-1384.
- Reddy PV, Shekar KC, Khandagale SB, Hara D, Dalip K, et al. (2019) Easy access to water‐ soluble cationic porphyrin‐β‐carboline conjugates as potent photocytotoxic and DNA cleaving agents. Asian. J Org Chem 8(2): 269-274.
- Tokala R, Thatikonda S, Vanteddu US, Sana S, Shankaraiah N, et al. (2018) Design and synthesis of DNA‐interactive β‐carboline–oxindole hybrids as cytotoxic and apoptosis‐inducing agents. Chem Med Chem 13(18): 1909-1922.
- Xu T, Shi L, Zhang Y, Wang K, Yang Z, et al. (2019) Synthesis and biological evaluation of marine alkaloid-oriented β-carboline analogues. Eur J Med Chem 168: 293- 300.
- Sathish M, Kavitha B, Nayak VL, Tangella Y, Ajitha A, et al. (2018) Synthesis of podophyllotoxin linked β-carboline congeners as potential anticancer agents and DNA topoisomerase II inhibitors. Eur J Med Chem 144: 557-571.
© 2020 Narayana Nagesh. This is an open access article distributed under the terms of the Creative Commons Attribution License , which permits unrestricted use, distribution, and build upon your work non-commercially.
 a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in
a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in 







.jpg)






























 Editorial Board Registrations
Editorial Board Registrations Submit your Article
Submit your Article Refer a Friend
Refer a Friend Advertise With Us
Advertise With Us
.jpg)






.jpg)














.bmp)
.jpg)
.png)
.jpg)










.jpg)






.png)

.png)



.png)






