- Submissions

Full Text
Modern Approaches in Drug Designing
Plant Extracts as Antiviral Agents
Jagessar RC
1Department of Chemistry, South America
*Corresponding author: Jagessar RC, Department of Chemistry, Faculty of Natural Sciences, Guyana, South America
Submission: June 06, 2020;Published: July 10, 2020

ISSN: 2576-9170 Volume3 Issue1
Abstract
With the advent of COVID-19, an infectious disease, caused by severe acute respiratory syndrome corona virus 2 (SARS-COV-2), which has claimed the death of thousands around the globe, there is an urgent intense need to screen plant extracts, in addition to the search for synthetic medicines and a vaccine to neutralize the coronavirus. Whilst the use of plant extracts for antimicrobial, antidiabetic and other assays have reached their zenith, research in the use of plant extracts as antiviral agents lacks comparison. Such research would also come in close scrutiny in the future, considering that the virus can mutate and lead to antiviral strains. Thus, research in this area, should seek the approval of health organisations, locally and international and July become increasing difficult to pursue, considering the emergence of COVID-19 disease. Viruses can mutate in the presence of chemicals and other mutating agents to produce Novel viral strains, with devastating effect on the human race. Viruses detrimental to the human race, can emerge from any countries. Once approval is sought, for antiviral research, herbal antiviral medicinal research should intensified. Guyana diverse flora offers a promising source for natural antiviral agents and needs continual screening in this direction. However, it would need approval from world health organisations for antiviral testing.
Keywords: COVID-19; SARS-COV-2; Antiviral; Antimicrobial; Viruses; Mutate
Introduction
Viruses are obligate intracellular parasites. They contain little more than bundles of gene strands of either RNA or DNA, and are surrounded by a lipid containing envelope, that is derived from the host cell membrane [1]. A complete virus particle, also known as a virion, consists of nucleic acids, surrounded by a protective coat of protein, the capsid. These are formed from proteinaceous capsomeres. The capsid is made from proteins, under the genetic instructions of the viral genome. Its shape serves as a basis for morphological distinction. In general, there are four (4) main morphological virus types: helical, icosahedral, prolate and envelope [2-5]. Viruses utilize the synthetic environment of the host cell, to propagate new viruses or replicate. This is unlike bacterial cells, which are free living entities, outside of the host. There seems to be a current dispute whether viruses are living or non-living outside of the host. Amongst the viruses that induce diseases are herpes simplex virus (HSV), cytomegalovirus (CMV), varicella zoster virus (VZV), hepatitis C virus (HCV), hepatitis B virus (HBV), influenza human immunodeficiency virus (HIV), respiratory syncytial virus (RSV) and recently, SARSCOV- 2. Viruses induce a wide range of diseases. These include Lassa fever, Ebola fever, AIDS, papillomavirus (HPV), infectious mononucleosis, mumps, measles and rubella, shingles, viral gastroenteritis (stomach flu), viral hepatitis, viral meningitis, viral pneumonia, common cold. These diseases appear to have no cure or vaccine to date [6,7].
Viruses have been successful as invasion hosts due to four attributes. These include genetic variation, variety in means of transmission, efficient replication within host cells and the ability to persist in the host1. In addition, viruses can mutate to a more resistant strain. The presence of mutating agents such as certain chemicals can cause this mutation. Each strain of virus has its own unique configuration of surface molecules [1]. The surface molecules work like lock and keys, enabling viruses to enter into hosts by precisely fitting the molecules on their surface to those on the membranes of target cells. In addition, viruses can change their conformation, as a result of mutation, making it difficult for a particular drug, which may show initial antiviral activity to become non-functional later. This makes it difficult to eradicate viruses. Hence, combination cocktail drug treatment has been used recently and has shown to be successful against viruses.
However, to date, there are few drugs or vaccine developed to eradicate viruses. To eradicate viruses, one has to know the life cycle of the virus, especially the critical steps. Viral replication involves several steps. Antiviral agents, both synthetic and of plant extracts origin can target any of these steps. Antiviral agents are expected to work via the inhibition of viral DNA or RNA synthesis or an inhibition of the activity of viral replication in the host environment or viral genome synthesis. Antiviral agents can get incorporated into viral DNA and causes DNA chain termination. Antiviral agents can be synthetic drugs, vaccines and plant extracts. Antiviral drugs can exert their actions at several stages of viral replication, including adsorption and penetration, nucleic acid synthesis, late protein synthesis, processing and in the final stages of viral packaging and virion release. Amongst the synthetic antiviral drugs currently in use are: Famciclovir, Penciclovir, Docosanol, Trifluride, Ganciclovir, Valganciclovir, Foscarnet, Cidofovir, Abacavir, Didanosine, Emtricitabine, Lamivudine, Stavudine, Tenofovir disoproxil fumarate, Zidovudine, Delavirdine, Efavirenz, Etravirine, Nevirapine, Rilpivirine, Atazavir, Darunavir, Fosamprenavir, Indinavir, Lopinavir, Nelfinavir, Ritonavir, Saquinavir, Tipranavir, Enfuvirtide, Maraviroc etc. These synthetic antiviral agents, which when administered can in addition to suppress the proliferation of the virus can cause irreversible side effects [7]. Thus, there is a need to use alternative or complementary medicines such as plant extracts.
Plant extracts, in their crude state or via the isolation and purification of natural products have been used as antimicrobial [8-22], antidiabetic [23-31], anticancer agents [32-44]. Isolated natural products from plants have provided the platform for the design and synthesis of drugs in the Pharmaceutical industry. Over 50% of modern drugs are derived from natural products or derivatized natural products. There is also an increase use of plant extracts as medicinal therapeutics. However, truly lacking in ant virology studies is the use of plant extracts as antiviral agents. Research in this direction has been progressing in vivo and in vitro and needs to be intensified, in comparison to antimicrobial studies or other bioassays. The same plant extracts can have different antiviral activity against RNA and DNA viruses. However, such research needs to be fast tract, considering the emergence of new viral strains such as which has been the cause of Covid-19 [45]. A wide range of natural products such as flavonoids, terpenoids, lignans, sulphides, polyphenolics, coumarins, saponins, furyl compounds, alkaloids, polyenes, thiophenes, proteins and peptides have been identified as possible antiviral agents [46-50]. In addition, selected essential oils of culinary herbs, spices and herbal teas have been shown to exhibit a significant level of antiviral activities [51,52]. Due to the high prevalence of viral infections, having no specific treatment and the constant appearance of resistant viral strains, the development of novel antiviral agents is essential. Figure 1 and Figure 2 show a list of natural products with promising antiviral activities and which should form the platform for other synthetic drugs mimicry.
Figure 1: Some natural products with promising antiviral activities.

Figure 2: Some natural products with promising antiviral activities.
There are reports of plant extracts being used as anti-viral agents. Several can be cited [53-58]. A study was carried out to investigate the antiviral screening activities of twenty seven (27) medicinal plant extracts, belonging to twenty six (26) different plant species from Nigerian ethnobotany, against echovirus 7, 13 and 19 serotypes (E7, E13 and E19, respectively [53]. Echoviruses infect millions of people globally and there is no specific drug treatment or vaccine available for its management currently. It was found that the crude extract of Macaranga barteri leaves had the highest cytotoxicity effect, followed by Crinum jagus and Terminalia ivorensis. Ten out of the twenty-seven crude plant extracts tested were active on E7 and E19, inhibiting the cytopathic effect of the virus in tissue culture. None of the extracts inhibited the cytopathic effect caused by E13 serotype. The methanol extract of M. barteri leaves had the highest antiviral activity on both E7 and E9, followed by the Ageratum conyzoides extract and Mondia whitei extract. Amongst the fractions of M. barteri, the DCM fraction was most the active and selective on E7.
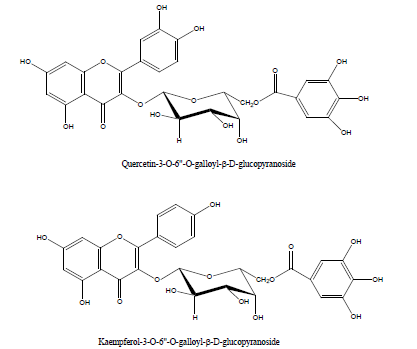
The evaluation of the in vitro anti-herpetic activity of twenty- five (25) Egyptian plants extracts against Herpes Simplex Virus type 1 was investigated on Vero cell lines by cell viability. Only two plants extracts; namely Euphorbia coopire (Euphorbiaceae) and Morus alba (Moraceae) showed potent anti-herpetic activity and six other extracts showed moderate inhibition. In contrast, a bioassay monitored phytochemical exploration of these two plants led to the isolation of pure flavonoid compounds. The antiviral activity of the isolated compounds was also examined. Seven pure compounds namely; 7-galloyl catechin, gallic acid, kaempferol 3-O-β-(6″-O-galloyl)- glucopyranoside, quercetin 3-O-β-(6″-O-galloyl)-glucopyranoside, curcumin, quercetin and kaempferol exhibited significant inhibition [54].
Ninety aqueous and hydroaloholic extracts from thirty-six (36) native plants of Chile and introduced plant species were screened for antiviral activity on herpes (HSV‐1 and HSV‐2) and HIV viruses. Furthermore, the samples were assayed for antimicrobial effect on pathogenic bacteria and a yeast. Plants were selected according to their indication of use for treating symptomatology of possible viral aetiology in Chilean folk medicine. The hydroaloholic extracts of Cassia stipulacea and Escallonia illintia exhibited detectable antiviral effects towards HSV‐1 with IC50 values of 80 and 40μg crude extract/mL, respectively. Samples belonging to Aristotelia chilensis (IC50 of 40μg/mL), Drymis winteri (IC50 values of 35 and 80μg/ mL), Elytropus chilensis and Luma apiculata, with an IC50 value of 100μg/mL showed activity against HSV‐2. None of the extracts showed activity against HIV at extract concentrations which were nontoxic for cells [56].
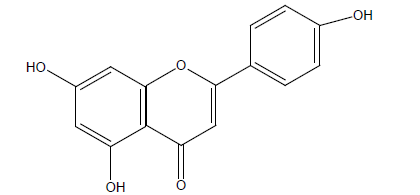
The organic and aqueous extract from Baccharis gaudichaudiana, B. spicata, Bidens subalternans, Pluchea sagitallis, Tagetes miauta and Tessaria absinthiodes were investigated for their antiviral activity against bovine viral diarrhea virus, herpes simplex virus type 1 (HSV-1), poliovirus type 2 (PV-2) and vesticular stomatitis. There was also a characterization of the antiviral activity of the organic and aqueous extract of B. gaudichaudiana. The bio-assay guided fractionation of the former led to the isolation of an active compound, apigenin: 5, 7-dihydroxy-2-(4-hydroxylphenyl)-4H – chromen-4-one [57] Figure 3.
Figure 3: Apigenin: 5, 7-dihydroxy-2-(4-hydroxylphenyl)- 4H –chromen-4-one, C15 H10 O5.
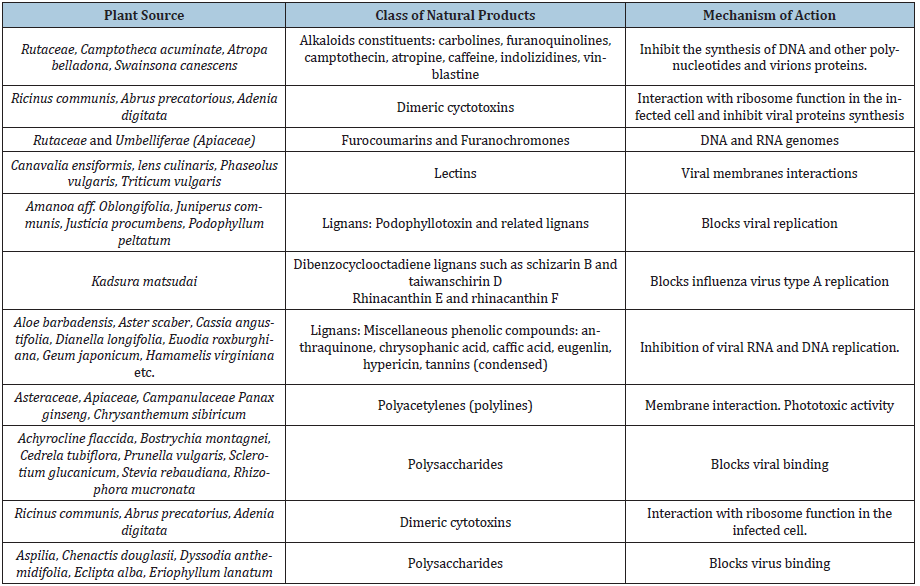
Selective antival activity was seen by the liquid root extract of Eleutherococcus senticosus. The root extract exhibited antiviral activity against the human rotavirus (HRV), RSV and influenza A virus. In constrast, the DNA viruses, adenovirus and HSV type 1 virus (HSV-1) were not inhibited by the extract. Thus, the antiviral activity of Eleutherococcus senticosus is viral dependent [58]. Table 1 gives a list of plant extracts tested positive for their antiviral activity. Also, their isolates.
Table 1: A list of plant extracts tested positive for their antiviral activity. Also, their isolates.
Conclusion
There is indeed an urgent continuing need to screen plant extracts for their antiviral activity in light of COVID-19. Also, the isolation and structural elucidation of the antiviral isolates which can form the platform for the synthesis of novel antiviral drugs. Plants antiviral activity may overlap with its antimicrobial, antidiabetic, anticancer activity, etc. The same class of natural products may have versatile medicinal roles. Current, synthetic antiviral drugs can be modified synthetically to study their antiviral structure activity relationship. Since viruses mutate, it seems as though combination cocktail drug treatment and the use of combined plant extracts may be the way forward for antiviral treatments. Antiviral research in any country will need approval from WHO in the future, as any virus in any country can mutate to a resistant strain, that could affect the entire world.
References
- Wagner EK, Hewlett MJ (1999) Basic virology. Blackwell Science, Malden, MA, USA.
- Casens S (2009) Desk encyclopedia of general virology. Boston, Academic Press, Cambridge, Massachusetts, USA, pp. 167-74.
- Caspar DL, Klug A (1962) Physical principles in the construction of regular viruses. Cold Spring Harbor Symposia on Quantitative Biology 27: 1-24.
- Crick FH, Watson JD (1956) Structure of small viruses. Nature 177 (4506): 473-475.
- Falvo MR, Washburn S, Superfine R, Finch M, Brooks FP, et al. (1997) Manipulation of individual viruses: Friction and mechanical properties. Biophysical Journal 72 (3): 1396-1403.
- Peter J (1994) Viruses on the edge of life, on the edge of death. National Geographic Magazine, pp. 58-91.
- Trevor, AJ, Katzung BG, Masters SB (2005) Katzung & Trevor Pharmacology Examination & Review. (7th edn), Lange Medical Books, McGraw-Hill, Pennsylvania Plaza, New York City, USA.
- Shahid W, Durrani R, Iram S, Durrani M, Khan F (2013) Antibacterial activity in vitro of medicinal plants. Sky Journal of Microbiology Research 1(2): 5-21.
- Rijo P, Faustino C, Simoes MF (2013) Antimicrobial natural products from Plectranthus plants, Microbial pathogens and strategies for combating them: Science, Technology and Education, pp. 922- 931.
- Arif T, Bhosale JD, Kumar N, Mandal TK, Bendre RS, et al. (2009) Natural products-antifungal agents derived from plants. Journal of Asian Natural Products Research 11 (7): 621-638.
- Melgarejo M, Mollinedo P, Castro JV (2006) Antibacterial activity of four natural products from a bolivian highland plant. Revista Boliviana De Quimica 23(1): 40-43.
- Saswati R, Choudhury MD, Paul SB (2013) In vitro antibacterial activity of Alocasia Decipiens Schott. International Journal of Pharmacy and Pharmaceutical Sciences 5(1): 155-157.
- Aarati N, Ranganath N, Soumya G, Kishore B, Mithun K (2011) Evaluation of antibacterial and anticandidal efficiency of aqueous and alcoholic extract of neem, Azadirachta indica. International Journal of Research and Pharmaceutical 2(1) 230-235.
- Kubde MS, Khadabadi SS, Saboo SS, Ghorpade DS, Modi AJ (2010) In vitro antimicrobial activity of the crude extracts of Colocasia Esculenta Leaves (Araceae). International Journal of Pharmacy and Pharmaceutical Sciences 1(8): 88-91.
- Jagessar RC, Mohamed N (2010) Antimicrobial activity of selected plants extracts from Guyana’s flora. Journal of Pure and Applied Microbiology 4(2): 533-540.
- Jagessar RC, Allen R (2011) Antimicrobial potency of the aqueous extract of leaves of Terminalia catappa. Academic Research International 1(3): 362-371.
- Jagessar RC, Mars A, Gomathigayam S (2011) Selective antimicrobial properties of leaf extract of Samanea Saman against Candida albicans, Staphylococcus aureus and Escherichia coli using several microbial techniques. Journal of American Science 7(3): 108-119.
- Jagessar RC, Mars A, Gomes G (2009) Leaf extract of Smilax schomburgkiana exhibit selective antimicrobial properties against pathogenic microorganisms. Life Science Journal 6(1): 76-83.
- Jagessar RC, Mohammed A, Gomes G (2008) An evaluation of the antibacterial and antifungal activity of leaf extracts of Momordica Charantia against Candida albicans, Staphylococcus aureus and Eschericia Coli. Nature and Science 6(1): 1-14.
- Jagessar RC, Ramchartar N, Spencer O (2015) A review of the antimicrobial activity of various solvent type extracts from fruits and edible plants, in “fruit and pomace extracts: Biological activity, potential applications and beneficial health effects. Nova Science Publisher.
- Jagessar RC, Hafeez A, Chichester M, Crepaul Y (2017) Antimicrobial activity of the ethanolic and aqueous extract of passion fruit (Passiflora edulis Sims), in the absence and presence of Zn (OAc)2.2H2 World Journal of Pharmacy and Pharmaceutical Sciences 6 (9): 230-246.
- Jagessar RC, Hope S (2016) Antimicrobial activity of the uncombined and combined aqueous extract of Phyllanthus Acidus, Sphagneticola Trilobata Leaves and Doliocarpus Dentatus Bark against Selective Pathogenic Microorganisms in the absence and presence of Zn2+ World Journal of Pharmacy and Pharmaceutical Sciences 5(8): 58-71.
- Jagessar RC, Rodriques A, Prasad K, Husain A, Kanhai V (2018) An investigation of the hypoglycemic effect of the aqueous extract of the fruits of Psidum Guajava, Averrhoa Bilimbi and the peel of Tamarindus indica in Normoglycemic guinea pigs. WJPPS 7(4): 77-101.
- Rai PK, Jaiswal D, Mehta S, Watal G (2009) Anti-hyperglycaemic potential of Psidium guajava raw fruit peel. Indian J Med Res 129(5): 561-565.
- Banu MS, Sridharan SK, Manikandan R (2013) Antihyperglycemic and antihyperlipidemic potentials of Psidium guajava in alloxan-induced diabetic rats. Asian Journal of Pharmaceutical and Clinical Research 6(1): 88-89.
- Bhutkar MA, Bhise SB (2011) Anti-oxidative effect of Tamarindus Indica in alloxan induced diabetic rats. International Journal of Research in Pharmaceutical and Biomedical Sciences 2(3): 1006-1009.
- Najmi A, Akhtar M, Mohd A, Mujeeb M, Pillai KK, et al. (2012) A pharmacological appraisal of medicinal plants with antidiabetic potential. Journal of Pharmacy and Bioallied Sciences 4(1): 27-42.
- Huang CS, Yin MC, Chiu LC (2011) Antihyperglycemic and antioxidant potential of Psidium guajava fruit in Streptozotozin induced diabetic rats. Food and Chemical Toxicology 49(9): 2189-2195.
- Bhutkar MA, Bhise SB (2011) Anti-oxidative effect of Tamarindus Indica in alloxan induced diabetic rats. International Journal of Research in Pharmaceutical and Biomedical Sciences 2(3): 1006-1009.
- Pushparaj P, Tan CH, Tan BK (2000) Effects of Averrhoa bilimbi leaf extract on blood glucose and lipids in streptozotocin-diabetic rats. Journal of Ethnopharmacology 72(1-2): 69-76.
- Surya B, Kurup S (2017) Averrhoa bilimbi fruits attenuate hyperglycemia-mediated oxidative stress in streptozotocin-induced diabetic rats. Journal of Food and Drug analysis 25(2): 360-368.
- Seidel C, Florean C, Schnekenburger M, Dicato M, Diederich M (2014) Plant-derived epigenetic modulators for cancer treatment and prevention. Biotechnology Advances 32(6): 1123-1132.
- Azmi AS, Bhat SH, Haniff S, Hadi SM (2006) Plant polyphenols mobilize endogenous copper in human peripheral lymphocytes, leading to oxidative DNA breakage: A putative mechanism for anticancer properties. FEBS Letters 580(2): 533-538.
- Apostolou A, Stagos D, Galitsiou E, Spyrou A, Haroutounian S, et al. (2013) Assessment of polyphenolic content, antioxidant activity, protection against ROS-induced DNA damage and anticancer activity of Viti vinifera stem extracts. Food and Chemical Toxicology 61: 60-68.
- Siriwantanmetanon N, Fiebich BL, Efferth T, Prieto JM, Heinrich M (2010) Traditionally used Thai medicinal plants: In vitro anti-inflammtory, anticancer and antioxidant activities. Journal of Ethnopharmacology 130(2): 197-207.
- Cao J, Xia X, Chen X, Xiao J, Wang Q (2013) Characterisation of flavonoids from Dryopteris erythrosora and evaluation of their antioxidant, anticancer and acetylcholinesterase inhibition activities. Food and Chemical Toxicology 51: 242-250.
- Wen L, Wu D, Jiang Y, Prasad KN, Lin S, et al. (2014) Identification of flavonoids in litchi (Litchi chinensis Soon.) leaf and evaluation of anticancer activities. Journal of Functional Foods 6: 555-563.
- Xia X, Cao J, Zheng Y, Wang Q, Xiao J (2014) Flavonoid concentrations and bioactivity of flavonoid extracts from 19 species of ferns from China. Industrial Crops and Products 58: 91-98.
- Malikovia J, Swaczyynova J, Kolar Z, Strnad M (2008) Anticancer and antiproliferative activity of natural brasinosteroids. Phytochemistry 69(2): 418-426.
- Steigerova J, Oklest kova J, levkova L, Kolar Z, Strnad M (2010) Brassinosteroids cause cell cycle arrest and apoptosis of human breast cancer cells. Chemico-Biological Interactions 188(3): 487-496.
- Cornblatt BS, Ye L, Dinkova-Kostova AT, Erb M, Fahey JW, et al. (2007) Preclinical and clinical evaluation of sulforaphane for chemoprevention in the breast. Carcinogenesis 28(7): 1485-1490.
- Amos LA, Lowe J (1999) How taxol stabilizes microtubule structure. Chemistry & Biology 6(3): 65-69.
- Jordan MA, Wilson L (2004) Microtubules as a target for anticancer drugs. Nature Reviews: Cancer 4(4): 253-266.
- Khazir J, Mir BA, PilcherL, Riley DL (2014) Rolke of plants in anticancer drug discovery. Phytochemistry Letters 7: 173-181.
- Solowey E, Lichtenstein M, Sallo S, Paavilainen H, Solowet E, et al. (2014) Evaluating medicinal plants for their anticancer effects. The Scientific World Journal 1-12.
- Chen N, Zhou M, Dong X, Qu J, Gong F, et al. (2020) Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: A descriptive study. Lancet 395(10223): 507-513.
- Hui DS, I Azhar E, Madani TA, Ntoumi F, Kock R, et al. (2020) The continuing 2019-nCoV epidemic threat of novel coronaviruses to global health, the latest 2019 novel coronavirus outbreak in Wuhan, China. International Journal of Infectious Diseases 91: 264-266.
- Sakagami H, Sakagami T, Takeda M (1995) Antiviral properties of polyphenols. Polyphenol Actualities 12: 30-32.
- Gauntt CJ, Wood HJ, McDaniel HR, McAnalley BH (2000) Aloe polymannose enchances anti-coxsackievirus antibody titres in mice. Phytotherapy Research 14(4): 261-266.
- Bunyapraphatsara N, Dechsree S, Yoosook C, Herunsalee A, Panpisutchai Y (2000) Anti-herpes simplex virus component isolated from Maclura cochinchinensis. Phytomedicine 6(6): 421-424.
- Dai JR, Hallock YF, Cardellina JH, Boyd MR (1998) HIV-Inhibitory and cytotoxic oligostilbenes from leaves of Hopea malibato. Journal of Natural Products 61(3): 351-353.
- Cox SD, Mann CM, Markham JL (2001) Interactions between components of the essential oil of Melaleuca alternifolia. Journal of Applied Microbiology 91(3): 492-497.
- Schnitzler P, Schon K, Reichling J (2001) Antiviral activity of Australian tea tree oil and eucalyptus oil against herpes simplex virus in cell cultures. Die Pharmazie 56(4): 343-347.
- Omonike O, Toluwanimi A, Peter S, Temitope F, Adekunle A (2018) In vitro antiviral activity of twenty-seven medicinal plant extracts from Southwest Nigeria against three serotypes of echoviruses. Virology Journal 15(1):110.
- El-Toumy SA, Salib JY, El-Kashak WA, Marty C, Bedoux G, et al. (2018) Antiviral effect of polyphenol rich plant extracts on herpes simplex virus type. Food Science and Human Wellness 7(1): 91-101.
- Pacheco P, Sierra JG, Schmeda‐Hirschmann, CW Potter, BM Jones, et al. (1993) Antiviral activity of chilean medicinal plant extracts. Phytotherapy Research 7(6): 415-418.
- Jaime, MFV, Redko F, Muschietti, LV, Campos RH, Martino VS, Cavallaro, LV (2013) In vitro antiviral activity of plant extracts from Asteraceae medicinal plants. Virology Journal 10: 245.
- Glatthaar-Saalmuller B, Sacher F, Esperester A (2001) Antiviral activity of an extract derived from roots of Eleutherococcus senticosus. Antiviral Research 50(3): 223-228.
© 2020 Ahmed M Abu-Dief. This is an open access article distributed under the terms of the Creative Commons Attribution License , which permits unrestricted use, distribution, and build upon your work non-commercially.
 a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in
a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in 







.jpg)






























 Editorial Board Registrations
Editorial Board Registrations Submit your Article
Submit your Article Refer a Friend
Refer a Friend Advertise With Us
Advertise With Us
.jpg)






.jpg)














.bmp)
.jpg)
.png)
.jpg)










.jpg)






.png)

.png)



.png)






