- Submissions

Full Text
Modern Approaches in Drug Designing
An Overview of Microsponge as a Novel Tool in Drug Delivery
Abdulla Ahmed1, Mina Makram1, Mostafa Sayed1 and Dina Louis1,2*
1Department of Pharmaceutics and Pharmaceutical Technology, Heliopolis University for Sustainable Development, Egypt
2Department of Pharmaceutics and Industrial Pharmacy, Cairo University, Egypt
*Corresponding author: Dina Louis, Department of Pharmaceutics and Pharmaceutical Technology, Cairo University, Egypt, Kasr Eleini Street
Submission: June 18, 2018;Published: August 01, 2018

ISSN: 2576-9170
Volume2 Issue3
Abstract
Micro sponges are polymeric delivery systems consisting of porous microspheres having a size range from 5 to 300 micron. Microsponge Delivery System (MDS) is a unique technology for controlled drug delivery. The present review introduces Microsponge technology along with its synthesis, characterization, parameters and release mechanism. Wide ranges of applications are also preferred to develop drug or cosmetic products with enhanced safety and efficacy. MDS can provide increased efficacy for topically active agents with improved safety, extended product stability and improved properties in anefficient and novel manner.
Keywords: Microsponge; Drug targeting; Porous nature; Topical delivery; Solvent diffusion; Polymerization
Abbreviations: MDS: Microsponge Delivery System; TEC: Triethylcitrate; DSC: Differential Scanning Colorimetry; TLC: Thin layer chromatography; FT-IR: Fourier Transform Infra-red spectroscopy; XRD: X-ray diffraction; ePTFE: Expanded Poly Tetra Fluoro Ethylene; PGA: Poly glycolic acid; PLLA: Poly-L-lactic Acid; dsRNA: Double stranded RNA; PEI: Poly Ethylene Imine; NTPs: Nucleoside Tri Phosphates
Introduction
Drug delivery systems that can specifically control the release rates and target drugs to specific locations of the body have a major impact on the health care system. Conventional formulations of topical drugs aim at working on the outer layers of the skin. Hence, after application, they release their active ingredients and deliver a high concentration of the active ingredient that is rapidly absorbed. However, they cause excessive accumulation of ingredients within the epidermis and the dermis. Therefore, it is required to have a system which extends the time during which an active ingredient is available either on skin surface or within the epidermis. In the meantime, such system has to decrease active ingredient penetration through the skin into the body. Lately, a new drug delivery system was discovered called “Microsponge”. The Microsponge Delivery System (MDS) is a highly cross-linked, patented polymeric system consisting of porous microspheres. They are small spongylike spherical particles consisting of an extremely large number of empty spaces which are connected to each other within a non-collapsible structure.
figure 1: Highly porous nature of a Microsponge.
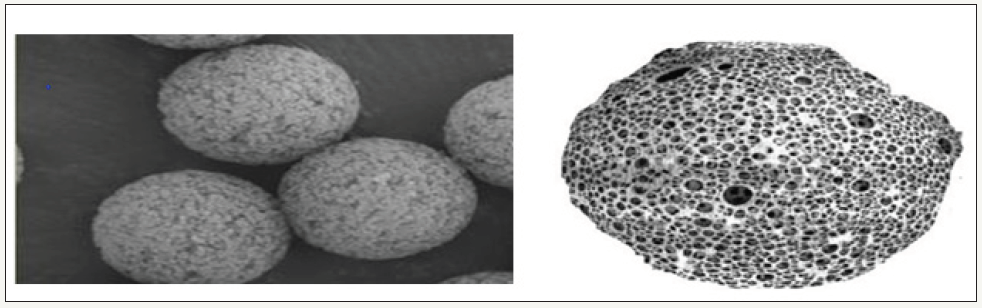
This large porous surface controls the release rate of active ingredient. The microsponge size varies from 5 to 300 μm in diameter and a typical 25μm sphere can have up to 250000 pores, (Figure 1). This results in having a large reservoir, which can be loaded with the active agent up to its own weight. So it has the flexibility to entrap a wide variety of active ingredients such as emollients, fragrances, sunscreens, essential oils, anti-infective, anti-fungal and anti-inflammatory agents and is used as a topical carrier system [1- 3].
History Of Microsponge
The microsponge technology was developed by Won in 1987 and the original patents were assigned to Advanced Polymer Systems, Inc. This Company developed variations of the technique and applied them to cosmetic as well as non-prescription drugs (OTC) and prescription pharmaceutical products. Now, this interesting technology has been licensed to Cardinal Health, Inc. for use in topical products [1,4].
Advantages of micro sponges over other formulations
Micro sponges have several advantages over other preparations available in the market.
Advantages over conventional formulations: Conventional formulations of topical drugs are aimed to work on the outer layers of the skin. Such products after application release their active ingredients. They deliver a layer of concentrated active ingredient that is rapidly absorbed. This results in excessive accumulation of ingredients within the epidermis and the dermis. Microsponges can prevent this. Possibly, the microsponge system can minimize significantly the side effect of drug like irritation without decreasing its efficacy through delivering the active ingredient gradually to the skin, like MDS Benzoyl peroxide formulations which have excellent efficacy with minimal irritation [5].
Advantages over microencapsulation and liposomes: The MDS has feature over other technologies like microencapsulation and liposomes. The rate of release of actives usually cannot be controlled in microcapsules. The actives contained within microcapsules will be released once the wall is ruptured. Liposomes suffer from limited capacity, difficult formulation, restricted chemical stability and microbial instability
Advantages over ointments: Patient compliance with ointment is reduced due to its aesthetically unattractive, viscous and greasy nature. Ointments have low efficiency as drug delivery systems, thus they cause irritation and sensitization because these compounds need high concentrations of active ingredients for effective treatment. Another drawback of topical formulations is the bad odor, uncontrolled evaporation of active ingredient and potential incompatibility of drugs with vehicles. The microsponge system, however, increases time during which an active resides either within epidermis or on skin surface. In the meantime, microsponge system decreases drug transdermal breakthrough into the body.
In general, advantages of microsponge drug delivery system can be summed up in what follows: [6,7]
- It gives continuous action and prolonged release up to 12 hours.
- Enhances product performance.
- Decreases irritation and increases patient compliance.
- Gives elegance to product.
- Can be incorporated into different formulations.
- Has good thermal, physical and chemical stability.
- Non-irritant, non-mutagenic, non-toxic and nonallergenic.
- Allows the inclusion of non-mixable substance.
- Converts fluids into powders to improve material handling.
- Improves drug bioavailability.
- Improves treatment efficiency
- Has the capability of absorbing of skin secretions up to 6 times its weight without changing its Physical appearance, therefore reducing the oiliness of the skin.
Potential features of microsponge drug delivery systems: [8-11]
- Have stability in pH extending from 1 to 11.
- Have stability at high temperatures up to 130°C.
- Have self-sterilization due to pore size 0.2 5μm which prevents penetration of bacteria, thus they not require addition of a preservative.
- Have high loading capacity ranging from 50 to 60%.
- Free flow properties and can be productive in relation to its cost.
- Offer good compatibility with different vehicles and ingredients.
Characteristics of Materials to be Entrapped in Micro sponges: [12,13]
Active ingredients which can be entrapped in microsponge can be incorporated into different products such as powders, creams, lotions, gels and soaps.
Some requirements must exist in material that will get entrapped in microsponge such as:
- It should not be water miscible or roughly only lightly soluble.
- It should be inert to monomers.
- During formulation, it should not raise the viscosity of the mixture.
- It should not cause the spherical structure of the micro Sponges to collapse.
- It should be fully miscible with the monomer as well as be able to make them miscible by adding a small amount of water immiscible solvent.
- Stable to polymerization catalysts and conditions.
- Materials that are entrapped in the vehicle must be of restricted solubility to avoid problems in cosmetic preparations. The vehicles might consume micro sponges before the application, if solubility is not restricted.
- The microsponge capacity and polymer design for materials must be optimized for desired release rate for a certain period of time.
Methods of preparation of microsponge
Microsponge drug delivery system can be prepared in two ways: one-step process or two-step process that is liquid-liquid suspension polymerization and quasi emulsion solvent diffusion techniques, respectively, that is based on physico-chemical properties of drug to be loaded [14].
figure 2: Microsponge preparation by liquid-liquid suspension polymerization [15].
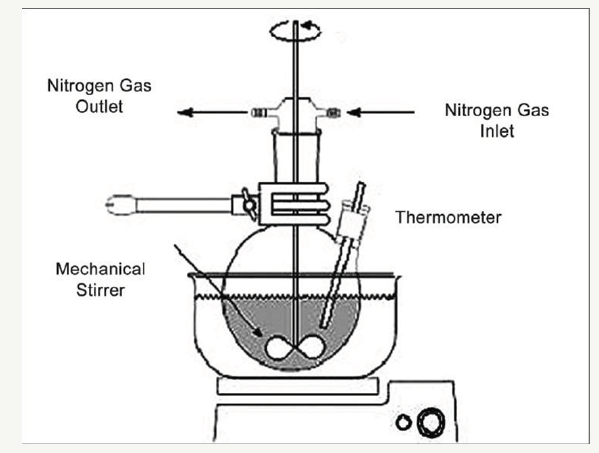
Liquid-liquid suspension polymerization
The porous microspheres are prepared by suspension polymerization method in liquid-liquid systems. In this method the monomers which are immiscible with each other, are first dissolved along with active ingredients in a suitable solvent, then are dispersed in the aqueous phase which consists of additives like surfactant or suspending agents to facilitate formation of suspension. The polymerization is then activated by increasing temperature, irradiation, or addition of a catalyst. The polymerization process results in the formation of a reservoir type of system with spherical structure. After the polymerization process, the solvent is removed leaving the microstructure, i.e., micro sponges (Figure 2) [15-17].
figure 3: Preparation of micro sponges by quasi emulsion solvent diffusion [13].

Quasi-emulsion solvent diffusion
Porous microspheres (micro sponges) were also prepared by a quasi-emulsion solvent diffusion method (two-step process) using an internal phase containing a polymer, such as eudragit, dissolved in ethyl alcohol. Then, the drug is slowly added to the polymer solution and dissolved under ultra-sonication at 350C. A plasticizer such as triethylcitrate (TEC) was added in order to aid the plasticity. The internal phase is then poured into an external phase containing polyvinyl alcohol and distilled water with continuous stirring for 2 hours. Then, the mixture was filtered to separate the micro sponges. The product (micro sponges) was washed and then dried in an air- heated oven at 40°C for 12 hours (Figure 3) [13-18].
Drug release mechanism from microsponge
The release of drug through micro sponges can be initiated by following triggers:
Solubility: Release can be achieved by diffusion taking into consideration the partition coefficient of the actives between micro sponges and outside system pH triggered system: The modification in coating of micro sponges can be used to achieve the pH-based drug release.
Pressure: The release of drug from micro sponges can be achieved by applying the pressure or by rubbing [19,20].
Temperature triggered system: The flow rate and release of the actives which are viscous at room temperature can be increased by increasing the skin temperature.
Evaluation of micro sponge
- Particle Size Determination: Microsponge particles should not be larger than 30 μm, and usually the particles range in size between 10 and 25 μm. Particle size analysis of loaded and unloaded micro sponges can be performed by laser light diffractometry or any other suitable Method [21].
- Morphology and Surface Topography of Micro sponges: Scanning electron microscopy (SEM) of a fractured microsponge particle can also be taken to illustrate its ultra structure [22].
- Determination of True Density: Microsponge density determination can be done using an ultra- pycnometer under helium gas and can be calculated from the mean of repeated determinations [23].
- Compatibility studies: These studies can be done through using thin layer chromatography (TLC), Fourier Transform Infra- red spectroscopy (FT-IR), powder X-ray diffraction (XRD) and Differential Scanning Colorimetry (DSC) [24].
- Polymer/Monomer Composition: Polymer composition of the MDS can affect partition coefficient of the entrapped drug between the vehicle and the microsponge system and hence have direct influence on the release rate of entrapped drug [23].
- Characterization of pore structure: Use mercury intrusion porosimetry [22].
- Resiliency (visco elastic properties): Resiliency of micro sponges can be studied and optimized as per the requirement by considering release as a function of cross-linking with time [24].
- Dissolution studies: This can be done Using dissolution apparatus USP XXIII with a modified basket consisting of 5μm stainless steel mesh [25].
Applications
Topical
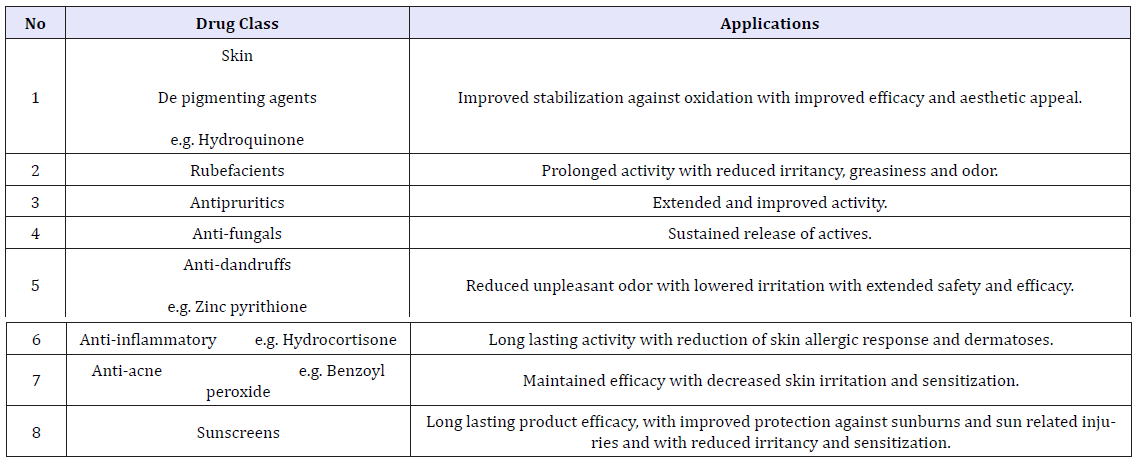
Many topical formulations are based on microsponge drug delivery system. Microsponge is polymer-based which allows binding or sustaining a large content of drug. This is due to its porous structure, where pore count reaches about 250000 pores in 1ml/g. This results in its capability of carrying drug more than any other drug delivery system, especially in topical formulations, as it increases the residence time of drug in the dermis or epidermis area. This allows for decreasing its prolonged use, decreasing the frequency, decreasing absorption into systemic circulation and decreasing its side effect on the skin as eczema, hypersensitivity and/or rash. Another way to prevent these side effects is using compatible, biologically inert polymer that the body does not identify as a foreign body. Also, the polymers used should be non-irritating, non- mutagenic, non-allergenic, non-toxic [26-32]; (Table 1).
Table 1: Applications of micro sponges for dermatological purposes.
Oral
The oral route is considered the most common route of administration due to its ease of access, high capability of dissolving Many drugs, and low toxicity. Although it is easy and safe, it is not compatible with all administered drugs, as drugs that have short life time that are excreted rapidly after administration, drugs that destroyed by the acidity of the stomach or bile juice secreted in the intestine, or drug that are needed to be in the colon to treat some medical condition. This gave rise to developing methods for controlling the release of the drug. Many drugs were formulated as microsponge that resulted in high advantages in this route of administration over nano particles, microspheres, liposomes, etc… In addition to its porous structure, micro sponges show longer lag time, which can promote colon targeting, through protecting the drug against stomach acidity and bile secretions in the small intestine as it only releases the drug at colon PH [10,33-36]; (Table 2).
Table 2: Oral applications of microsponge.

Future aspects
Tissue engineering:Tissue engineering is one of the most promising fields, as scientists believe that it can enhance quality of life of patients. One example is Vascular Tissue Engineering. The traditional way for it, is using expanded polytetrafluoroethylene (ePTFE) or Dacron that is clinically used for reconstructing large-diameter arteries, such as the aorta and iliac artery. They are not suitable for small artery as they are considered as foreign bodies resulting in thrombosis. Hence bypass procedure is required, which necessitates more surgical intervention. Through Scaffold Design technique, a newly designed small-caliber vascular graft made of polyglycolic acid (PGA) and poly-L-lactic acid (PLLA) fibers which were compounded with collagen microsponge to form a vascular patch material and tested on dogs. These dogs were totally recovered with no adverse events and no administration of anti-thrombotic drugs as this graft was made of biodegradable polymers that did not need pretreatment in the form of cell seeding, thereby conferring better patency on small-diameter vascular grafts [37-41].
RNAi:RNA interference, or RNAi, is thought to be a natural defense mechanism that has evolved for the protection of organisms from RNA viruses and cancer. Cells can recognize double stranded RNA (dsRNA) as an intruder. When this happens, the enzyme Dicer is recruited to cut the foreign RNA into smaller pieces called siRNA. These RNA pieces consist of approximately 22 nucleotides in length. One strand of the siRNA then binds to a target viral mRNA in a sequence-specific manner to interfere with its expression. There are many challenges that face the delivery of siRNA to the target site as:
- Administrative Barrier: as it cannot be available by oral route due to its insufficient permeability across intestinal epithelium into circulation.
- Immune Response and Safety: as its carrier need to be non-immunogenic, in order to prevent being destroyed before reaching the target.
- Delivering sufficient dose without the need for frequent doses.
Due to previous challenges, microsponge was considered to be the appropriate delivery system to overcome these challenges by using rolling circle transcription of a DNA template technique. The RNA polymers are generated by rolling circle transcription of circular DNA in a solution containing the RNA-producing enzyme RNA polymerase and RNA building blocks (nucleoside tri phosphates, NTPs). Then entangled and twisted fibrils, lamellar sheets and wrinkled sheets with semi-spherical structures are formed. Finally, the addition of poly cationic poly ethylenimine (PEI) causes the RNAi micro sponges to condense into 200-nmdiameter PEI-coated micro sponges containing approximately 500,000 siRNA precursors each (Figure 4) [42,43].
figure 4: Schematic outlining the one-pot synthesis of polymers of short double-stranded RNA (dsRNA) hairpins and their spontaneous assembly, over approximately 20 h, into 2-μm-diameter RNAi micro sponges through a series of intermediate [42].

References
- Jadhav N, Vruti P, Siddesh M, Gaurav B, Manisha K, et al. (2013) Microsponge delivery system: an updated review, current status and future prospects. Journal of Scientific and Innovative Research 2(6): 1097-1110.
- Embil K, Nacht S (1996) The microsponge® delivery system (MDS): A topical delivery system with reduced irritancy incorporating multiple triggering mechanisms for the release of actives. Journal of microencapsulation 13(5): 575-588.
- Nacht S, Katz M (1990) The Microsponge: A Novel Topical Programmable Delivery System. Drugs and the pharmaceutical sciences 42: 299-325.
- Won R (1987) Method for delivering an active ingredient by controlled time release utilizing a novel delivery vehicle which can be prepared by a process utilizing the active ingredient as a porogen. Google Patents.
- Kaity S, Sabyasachi M, Ashoke KG, Dilipkumar P, Animesh G, et al. (2010) Microsponges: A novel strategy for drug delivery system. J Adv Pharm Technol Res 1(3): 283-290.
- Aloorkar N, Kulkarni As, Ingale DJ, Patil RA (2012) Microsponges as innovative drug delivery systems. Int J Pharm Sci Nanotechnol 5(1): 1597-1606.
- D’souza JI, More HN (2008) Topical anti-inflammatory gels of fluocinolone acetonide entrapped in eudragit based microsponge delivery system. Res J Pharm Tech 1(4): 502-506.
- Aldawsari H, Badr Eldin SM (2013) Microsponges as promising vehicle for drug delivery and targeting: Preparation characterization and applications. African Journal of Pharmacy and Pharmacology 7(17): 873-881.
- Aritomi H, Yamasaki Y, Yamada K (1996) Development of Sustained- Release Formulation of Chlorpheniramine Maleate Using Powder-Coated Microsponge Prepared by Dry Impact Blending Method. Pharmacology 56(1): 49-56.
- Jain V, Singh R (2011) Design and characterization of colon-specific drug delivery system containing paracetamol microsponges. Arch Pharm Res 34(5): 733-740.
- Kawashima Y, Toshiyuki NIWA, Hirofumi, Tomoaki, Yoji ITO (1992) Control Of Prolonged Drug Release And Compression Properties Of Ibuprofen Microsponges With Acrylic Polymer Eudragit RS by Changing their Intraparticle Porosity. Chemical and pharmaceutical bulletin 40(1): 196-201
- Jain N, Sharma PK, Banik A (2011) Recent advances on microsponge delivery system. International Journal of Pharmaceutical Sciences Review and Research 8(2): 13-23.
- Charde M (2013) Microsponge: A novel new drug delivery system: A review. International Journal of Advances in Pharmaceutics 2(6): 63-70.
- Nanda S (2013) Microsponge drug delivery system: an overview. World Journal of Pharmacy and Pharmaceutical Sciences 2(3): 1032-1043.
- Çomoğlu T N, Gönül, T Baykara (2003) Preparation and in vitro evaluation of modified release Ketoprophen microsponge. Il Farmaco 58(2): 101-106.
- Khopade A, Jain S, Jain N (1996) The microsponge. Eastern pharmacist 39(459): 49-53.
- Potts R,Guzek DB, Harris RR, Mc Kie JE (1985) A noninvasive, in vivo technique to quantitatively measure water concentration of the stratum corneum using attenuated total-reflectance infrared spectroscopy. Archives of dermatological research 277(6): 489-495.
- D’Emanuele A, Dinarvand R (1995) Preparation, characterisation and drug release from thermo responsive microspheres. International Journal of Pharmaceutics 118(2): 237-242.
- Barkai A, Pathak Y, Benita S (1990) Polyacrylate (Eudragit retard) microspheres for oral controlled release of nifedipine I Formulation design and process optimization. Drug Development and Industrial Pharmacy 16(13): 2057-2075.
- D’souza JI, M Harinath (2008) The microsponge drug delivery system: for delivering an active ingredient by controlled time release. Pharma info net 6(3): 62.
- Disouza J (2001) In-vitro antibacterial and skin irritation studies of micro sponges of benzoyl peroxide. Indian Drugs-Bombay 38(7): 361- 362.
- Amrutiya N, A Bajaj, M Madan (2009) Development of micro sponges for topical delivery of mupirocin. AAPS PharmSciTech 10(2): 402-409.
- Barkai A, Y Pathak, S Benita (1991) Polyacrylate (Eudragit retard) microspheres for oral controlled release of nifedipine: formulation design and process optimization. Ellis Horwood Series In Pharmaceutical Technology 103.
- Li SS, Li GF, Liu L, Jiang X, Zhang B, et al. (2013) Evaluation of paeonol skin-target delivery from its microsponge formulation: in vitro skin permeation and in vivo micro dialysis. PloS one 8(11): e79881.
- Moin A, Tamal K, Riyaz Ali, Rohit R, Umme Hani, et al. (2016) Fabrication, characterization, and evaluation of microsponge delivery system for facilitated fungal therapy. Journal of basic and clinical pharmacy 7(2): 39-48.
- Osmani RAM (2015) Microsponges based novel drug delivery system for augmented arthritis therapy. Saudi Pharmaceutical Journal 23(5): 562- 572.
- Rizkalla CMZ, R latif Aziz, Soliman II (2011) In vitro and in vivo evaluation of hydroxyzine hydrochloride micro sponges for topical delivery. AAPS PharmSciTech 12(3): 989-1001.
- Gupta A, Tiwari G, Tiwari R, Srivastava R (2015) Factorial designed 5-fluorouracil-loaded micro sponges and calcium pectinate beads plugged in hydroxypropyl methylcellulose capsules for colorectal cancer. International journal of pharmaceutical investigation 5(4): 234-246.
- Khan MA (2015) Product development studies on sono crystallized curcumin for the treatment of gastric cancer. Pharmaceutics 7(2): 43-63
- Osmani RAM, Nagesh H, Aloorkar Bharati UT, hawareb Parthasarathi K, et al. (2015) Microsponge based drug delivery system for augmented gastro paresis therapy: Formulation development and evaluation. Asian journal of pharmaceutical sciences 10(5): 442-451.
- Aibibu D, Hild M, Wöltje M, Cherif C (2016) Textile cell-free scaffolds for in situ tissue engineering applications. Journal of Materials Science Materials in Medicine 27(3): 63.
- Iwai S, Sawa Y, Ichikawa H, Taketani S, Uchimura E, et al. (2004) Biodegradable polymer with collagen microsponge serves as a new bioengineered cardiovascular prosthesis. The Journal of Thoracic and Cardiovascular Surgery 128(3): 472-479.
- Iwai S, Sawa Y, Taketani S, Torikai K, Hirakawa K, Matsuda et al. (2005) Novel tissue-engineered biodegradable material for reconstruction of vascular wall. The Annuals of thoracic surgery 80(5): 1821-1827.
- Mendelson K, FJ Schoen (2006) Heart valve tissue engineering: concepts approaches progress and challenges. Annals of biomedical engineering 34(12): 1799-1819.
- Yokota T, Ichikawa H, Matsumiya G, Kuratani T, Sakaguchi T, et al. (2008) In situ tissue regeneration using a novel tissue-engineered, smallcaliber vascular graft without cell seeding. The Journal of thoracic and cardiovascular surgery 136(4): 900-907.
- Grabow WW, L Jaeger (2012) siRNA delivery: Loaded-up Microsponges. Nature materials 11(4): 268-269.
- Gu L, (2017) A Combination RNAi-Chemotherapy Layer by Layer Nano particle for Systemic Targeting of KRAS/P53 with Cisplatin to Treat Non–Small Cell Lung Cancer. Clinical Cancer Research 23(23): 7312- 7323.
- Han S, H Kim, JB Lee (2017) Library siRNA-generating RNA nano sponges for gene silencing by complementary rolling circle transcription. Scientific Reports 7(1): 10005.
- Lee JB, Jinkee Hong, Daniel K, Bonner, Zhiyong (2012) Self-assembled RNA interference micro sponges for efficient siRNA delivery. Nature materials 11(4): 316-322.
- Roh YH, Deng JZ, Dreaden EC, Park JH, Yun DS, et al. (2016) A multi‐RNAi microsponge platform for simultaneous controlled delivery of multiple small interfering RNAs. Angewandte Chemie International Edition 55(10): 3347-3351.
- Roh YH, Lee JB, Shopsowitz KE, Dreaden EC, Morton SW, et al. (2014) Layer-by-layer assembled antisense DNA microsponge particles for efficient delivery of cancer therapeutics. ACS nano 8(10): 9767-9780.
- Shopsowitz KE, Young H, Zhou J D, Stephen, Paula T (2014) Composite RNAi‐Microsponges Form through Self Assembly of the Organic and Inorganic Products of Transcription. Small 10(8): 1623-1633.
- Tatiparti K, Sau S, Kashaw SK, Iyer AK (2017) SiRNA delivery strategies: a comprehensive review of recent developments. Nano materials 7(4): 77.
© 2018 Dina Louis. This is an open access article distributed under the terms of the Creative Commons Attribution License , which permits unrestricted use, distribution, and build upon your work non-commercially.
 a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in
a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in 







.jpg)






























 Editorial Board Registrations
Editorial Board Registrations Submit your Article
Submit your Article Refer a Friend
Refer a Friend Advertise With Us
Advertise With Us
.jpg)






.jpg)














.bmp)
.jpg)
.png)
.jpg)










.jpg)






.png)

.png)



.png)






