- Submissions

Full Text
Modern Approaches in Drug Designing
Thiazolotriazole: An Emerging Novel Bridge Heterocycle with Medicinal Value
Nilesh A Karande* and Lalit G Rathi
Department of Pharmaceutical Chemistry, Institute of Pharmaceutical Education and Research, Wardha, India
*Corresponding author: Nilesh A Karande, Department of Pharmaceutical Chemistry, Institute of Pharmaceutical Education and Research, India
Submission: February 21, 2018; Published: March 13, 2018

ISSN : 2576-9170Volume1 Issue5
Opinion
In lieu of more than a century, heterocycles have constituted one of the prevalent areas of research in organic chemistry. Heterocycles play an imperative part in biochemical processes since the flank groups of the most emblematic and vital parts of living cells, DNA as well as RNA are based on aromatic heterocycles. Amid nearly 20 million chemical compounds acknowledged by the end of the second millennium, more than two-third is completely or moderately aromatic and almost half are heterocyclic. The occurrence of heterocycles in all kind of organic compounds of curiosity in biology, pharmacology, optics, electronics, material sciences and so on are very well notorious. Flanked by sulphur and nitrogen having heterocyclic compounds have conserved the curiosity of researchers over decades of historical development of organic compounds. The occurrence of heteroatoms results in substantial vicissitudes in the cyclic molecular structure owing to the obtainability of unshared pair of electrons and the alteration in electronegativity amongst heteroatoms and carbon. As a result nitrogen and sulphur heterocycles show physiochemical features and reactivity relatively different from the parent aromatic hydrocarbons [1].
In recent times copious attention has been schemed to the synthesis of thiazolo-1,2,4-triazoles owed to the veracity that they have a broad spectrum of biological activities such as antimicrobial, analgesic, anti-inflammatory, antipyretic, anticancer, and vasodilatory. Sulphur having heterocycles represent an important group of compounds that are favourable for use in practical applications. On the other hand the electronic structure of sulphur instils sulphurous organic compounds, through chemical reactivity beyond those of the corresponding oxygen or nitrogen holding analogues [2]. The curiosity in the [5,5]-fused bicycle as thiazolotriazoles for use in pharmaceutical products makes these gallows extremely expedient building block for organic chemistry Such spinoffs have found applications in oncology, infectiology or neurodegenerative diseases (Figure 1).
Thiazoles are the members of azole heterocycles thru bigger pi- electron delocalization than the subsequent oxazoles and have so loftier aromaticity. This aromaticity is demonstrated through the chemical shift of the ring protons in proton NMR spectroscopy analysis flanked by 7.27 and 8.77ppm, noticeably viewing a prevailing diamagnetic ring current. The accounted pi-electron density directs C5 as the focal place for electrophilic replacement, and C2 as the place for nucleophilic substitution [3]. 1,2,4-Triazole is a distinct pair of isomeric chemical compounds with molecular formula C2H3N3, which can be prepared through the Einhorn- Brunner reaction or the Pellizzari reaction. Encouraged from therapeutic importance of thiazole and triazole as a part of research in the area, it is reflection of interest to combine these two vital rings together.
Figure 1: Two isomers of thiazolo-1,2,4-triazole.
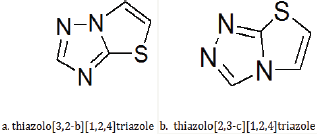
Conversely, the synthetic tools for retrieving highly functionalized thiazolotriazoles are very restricted and merely few functionalization approaches are described in the literature. In order to access to novel families of thiazolo[3,2-b][1,2,4]triazoles, there is consequently marvellous curiosity in developing efficient synthetic methodologies. In order to introduce a varied range of functional groups, a promising answer is to treasure an effective substitute to selectively functionalize thiazolo[3,2-b] [1,2,4] triazoles at the C-2 position [4].
Thiazolo[3,2-b]-1,2,4-triazoles can be prepared thru different ways where the vital techniques are alkylation of 3(5)-mercapto- 1,2,4-triazoles with phenacyl halides; chloroacetic acid or 1,2-dihaloethane; cyclization of 3-allyl-1,2,4-triazole with iodine; reactions of 3,5-dibromo-1-(thiiran-2-ylmethyl)-1,2,4-triazole with certain nucleophiles such as primary and secondary aliphatic amines; furthermore with primary aromatic amines [5].
Crystal structure studies of 6-Benzylidene-2-(2-chlorophenyl) thiazolo-[3,2-b]-l,2,4-triazol-5(6H)-one publicised that the glued thiazolo[3,2-b]-1,2,4-triazole system is nearly planar. The phenyl substituents are planar within investigational error and mark dihedral angles of 16.5(1) and 10.9(1)�x00B0; with the thiazolo triazole system. There remained three intramolecular H-bond interactions, C-H...N, C-H...O and C-H...S [6].
The superfluity of research signposts a wide spectrum of pharmacological activities revealed by thiazolotriazole derivatives. The biological profiles of these new cohorts of thiazolotriazoles would represent a prolific milieu for further development of better therapeutic agents. It can act as a key tool for medicinal chemists to develop novel compounds owning thiazolotriazole moiety that could be better agents in terms of efficacy and safety.
References
- Lu SM, Alper H (2005) Intramolecular carbonylation reactions with recyclable palladium-complexed dendrimers on silica: synthesis ofoxygen, Nitrogen, or sulfur-containing medium ring fused heterocycles. J Ame Chem Soc 127(42): 14776-14784.
- Karthikeyan MS (2009) Synthesis, analgesic, anti-inflammatory and antimicrobial studies of 2,4-dichloro-5-fluorophenyl containing thiazolotriazoles. Eur J Med Chem 44(2): 827-833.
- Gaware VM, Dighe NS, Pattan SR, Shinde HV, Musmade DS, et al. (2010) Thiazolo-Triazole a nucleus possessing range of pharmacological activities. Pharm Lett 2(2): 35-40.
- Erkhitueva EB, Dogadina AV, Khramchikhin AV, Ionin BI (2012) Highly regioselective heterocyclization reactions of 1H-1,2,4-triazole-3-thiols with chloroacetylenephosphonates. Tetrahedron Lett 53(33): 43044308.
- Abdel-Wahab BF, Mohamed HA (2014) Direct routes to thiazolotriazoles by cyclization. Phosphorus Sulfur Silicon Relat Elem 189(2): 157-179.
- Ozbey S, Kendi E, Tozkoparan B, Ertan M (1999) 6-Benzylidene-2-(2- chlorophenyl) thiazolo [3,2-b]-1,2,4-triazol-5(6H)-one. Acta Crystallogr C 55(11): 1939-1941.
© 2018 Nilesh A Karande, et al. This is an open access article distributed under the terms of the Creative Commons Attribution License , which permits unrestricted use, distribution, and build upon your work non-commercially.
 a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in
a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in 







.jpg)






























 Editorial Board Registrations
Editorial Board Registrations Submit your Article
Submit your Article Refer a Friend
Refer a Friend Advertise With Us
Advertise With Us
.jpg)






.jpg)














.bmp)
.jpg)
.png)
.jpg)










.jpg)






.png)

.png)



.png)






