- Submissions

Full Text
Journal of Biotechnology & Bioresearch
Antifungal Activity of M. indica and F. platyphylla Leaf and Stem Bark Extracts against Onion Bulb Rot Fungi: A Comparative Study in North Western Nigeria
Sunusi Bataiya Buhari1*, Ahmad Ibrahim2, Auwalu Aliyu3, Aliyu U Maaji4 and Labaran B Umar1
1Department of Biological Sciences, Yusuf Maitama Sule University Kano, Nigeria
2Department of Biochemistry, Federal University Lokoja, Kogi State
3Department of Biological Sciences, Federal university Dutse; Jigawa State, Nigeria
4Department of Biotechnology, Mewar university, India
*Corresponding author:Sunusi Bataiya Buhari, Department of Biological Sciences, Yusuf Maitama Sule University Kano, Nigeria
Submission: December 24, 2023;Published: January 26, 2024

Volume5 Issue2January 26, 2024
Abstract
In recent decades, there have been concerns of onion bulbs diseases due to microbial infection. Despite advancements in science and technology leading to the discovery and development of several quantitative control strategies, onion rots remain the leading cause of onion spoilage, especially in underdeveloped countries. This study aimed to investigate the antifungal activity of leaf and stem extracts from M. indica and F. platyphylla against fungi associated with rot diseases in onion in Kano state, Nigeria. Phytochemical analysis was conducted using standard methods to investigate the presence of saponin, glycoside, steroid, and phenol, alkaloid and flavonoid in the selected plants. Also the fungi associated with the rot of onion were isolated and identified using microscopic technique for their respective morphological features. This was followed by determination of inhibitory activity of the extracts against the identified fungi. The survey identified Aspergillus niger and Aspergillus flavus as the pathogenic fungi responsible for the rot disease and indicated that M. indica exhibited higher antifungal efficacy compared to F. platyphylla, with M. indica showing significant inhibition of both A. niger and A. flavus. The chloroform extract of M. indica leaf displayed the highest effectiveness, with 94.54% inhibition of A. niger and 93.63% inhibition of A. flavus. Additionally, the methanol extract of M. indica stem bark also exhibited notable antifungal activity. In contrast, F. platyphylla showed moderate to the least effective inhibition against the tested fungi. The findings suggest that both M. indica and F. platyphylla possess antifungal activity against A. niger and A. flavus in vitro.
Keywords:M. indica; F. platyphylla allium cepa; Aspergillus niger; Aspergillus flavus
Introduction
Onion is an important vegetable cash crop used in diets as seasoning, flavoring and medicinal purposes across cultures. It has since become an indispensable source of income for farmers across the globe. In Nigeria, onion is cultivated in several northwestern part of country including Kano State. However, most of these farmers sell off their product immediately after the harvest. This is majorly due to poor storage facilities resulting into microbial infestation and particularly onion rot caused by fungi contributing more to the damage [1,2]. Traditional and conventional methods to control these infections have not been effective as they have numerous limitations broadly categorized into human health and environmental pollution. Therefore, there is a need for naturally occurring management strategies to replace the inefficient methods currently being applied. Phytochemicals are biologically active, naturally occurring chemical compounds found in plants, which provide health benefits for humans including protection of plants from diseases and preserving their physicochemical conditions [3]. Phytochemicals have been classified as primary or as secondary metabolites depending on their role in plant metabolism [4]. Various properties have been attributed to plants as reservoirs of bioactive compounds for numerous applications. These bioactive compounds can be derived from any part of the plant including the leaf and stem [5]. Several studies have shown higher biological activities for the crude plant extracts than a single isolated compound due to their synergistic effects (Manoharachary and Nagaraju, 2016).
Plant products have been a part of antifungal compounds since ancient time and thus screening various plants for antifungal activity has been reported by Amos et al. [6]. Antifungal compounds naturally occur in plants, leaves vegetables, and roots with quantitative resistance, possessing defense mechanisms that protect against various plant diseases [7,5]. Antifungal screening of plants has revealed the presence of numerous bioactive compounds ranging from proteinaceous and non-proteinaceous metabolites which include thionine, defensins, flavonoid, phenols, terpenoids etc., [6-8]. These metabolites serve as defense mechanisms against microorganisms particularly for bacteria, parasites and fungi [8]. Therefore, the use of these management strategies have become more popular in the control of many plant diseases due to the widely held belief that it is safe. Mangifera indica L. and Ficus platyphylla extracts are quantitative resistance that are widely used to treat various plant diseases, particularly those causing onion rotting such as white rot, neck rot, soft rot purple blotch, blast, rust, smudge, leaf spot, pink rot, and basal rot etc., [9,10-12]. Terpenoids and alkaloids exhibit various important antifungal compounds used against onion rods. In view of that, this study aimed at analyzing the presence or absence of different antifungal compounds of M. indica and F. platyphylla and testing their effect in the control of fungi associated with onion rots in Kano state of Nigeria.
Method and Materials
Study sites
The experiments were conducted in the biological science department laboratory at Yusuf Maitama Sule University, Kano. Kano is located in the dry sub-humid agroecological zone with coordinates 11.58°39”N and 8.33°45”E. The annual rainfall ranges from 696.4mm to 700mm (27.4in) or 58mm (2.3in) per month. The wettest (rainy) weather is in August when an average of 228mm (9in) of rainfall occurs. The driest weather is in January, November and December when an average of 0mm (0in) of rainfall occurs. The hottest month is April with a maximum temperature of 38 °C (100°F), while the coldest month is December with an average maximum temperature of 29 °C (84°F). The mean annual temperature ranges from 26 °C to 32 °C.
Collection and authentication of plant sample
The fresh leaves and barks of M. indica and F. platyphylla were carefully collected from their natural habitat in the school farm of Yusuf Maitama Sule University. The samples of the plants’ leaves and barks in a polyethylene bag were conveyed to the herbarium section of Yusuf Maitama Sule University for taxonomic identification and authentication. The plant materials were brought to the laboratory, rinsed with water to remove dirt, and dried at room temperature before examination. The leaves and barks were then removed from the dried leaves and crushed into a powder form using a pestle and mortar.
Collection of onion bulb
The samples of the diseased onion bulbs were collected around Yusuf Maitama Sule University Market within Kano metropolis. The diseased onion bulbs were washed with tap water and surfacesterilized in 90% alcohol for 3 to 5 minutes, then rinsed with sterile distilled water before use.
Isolation and identification of onion bulb rot fungi
Onion bulb was used for the detection of the pathogens responsible for the rots on the affected onion bulbs. The bulb was stripped of its outer dry scale and surface-sterilized in 1% commercial bleach for one minute. It was then rinsed in three successive changes of sterile distilled water and blot dried with sterile filter paper. Small segments of tissues (3cm^3) from the advancing margins of rotted lesions were cut out with a sterile scalpel and forceps and plated on acidified Potato Dextrose Agar (PDA) in 90mm Petri dishes. The plates were incubated at room temperature (28±°C) for seven days. Developing fungal colonies were sub-cultured continuously on fresh PDA plates to obtain pure cultures of the isolates. Fungal isolates were microscopically examined and identified using a microscope, following identification guides based on cultural and morphological characteristics of the International Mycological Institute.
Preparation of the slide
Slides of the mycelium observed from different isolates were prepared as follows: A drop of lactophenol cotton blue solution was placed in the center of a clean glass slide. A small portion of the unidentified fungi culture was cut out with an inoculating needle; the portion was placed in the lactophenol cotton blue droplet on the slide and teased out with another needle. A cover slip was then lowered over the teased portion.
Identification of isolated fungi
Slides prepared from the individual fungal colonies were examined under the microscope to study their morphological features. The identified fungi were compared with the observed features of the colony descriptions by Robbert and Ellen [13].
Preparation of plant extract
50g of air-dried and ground Mangifera indica and Ficus platyphylla leaves and stem barks powder were extracted by percolation with 200ml (1:4 ratio) each of methanol and chloroform at room temperature for one week. The contents were filtered using Whatman No. 1 filter paper, and the residue was discarded. Later, the extracts were obtained following evaporation to dryness using a rotary evaporator.
Phytochemical analysis of plant extract
Phytochemical analysis was conducted to qualitatively determine the presence or absent of the following secondary metabolites that is alkaloid, glycoside, phenol, steroid, saponin, flavonoid, and carbohydrate. Using method outline by (Evans and Trease 1999).
Alkaloids: Using pipette, 3ml of drag end off reagent was added to the extract, forming creamy precipitate indicated the presence of alkaloid as reported by Evans and Trease (1999).
Tannins: Few drops of FeCl2 solution was added to 3ml of the extracts in a test tube followed by shaking. A result of dirty green coloration confirmed the presence of tannins as demonstrated by Evans and Trease (1999).
Flavonoids: One ml of the extract was treated with 1ml of dilute NaOH. The presence of a cloudy precipitate confirm the presence of flavonoid as described Evans and Trease (1999).
Saponin: Five milliliters(5ml) of distilled water was added to the 2ml of the extract in a test tube and shaken vigorously. The formation of foams or stable thing following the shaking indicated the presence of saponin as demonstrated by Evans and Trease (1999).
Phenol: One ml(1ml) of the extract was added to 1ml of FeCl3 and mix together. The presence of blue black precipitate confirmed the presence of phenols as described Evans and Trease (1999).
Glycoside: Approximately 2ml of glacial acetic acid were added to 5ml of the extract. Followed by one (1) drop of FeCl2 and concentrated H2S04. Brown ring precipitate indicated the presence of glycoside.
Result
Phytochemical screening
The results of the phytochemical analysis in each of the extracts (Figure 1) showed the presence of steroids, saponins and glycoside in both methanolic and chloroform of leaf extracts in M. indica. However, both extracts do not contain alkaloids nor flavonoids, but the chloroform extract contains an additional phytochemical, phenol (Table 1). Similarly, the stem bark extracts have the same composition of secondary metabolites as the leaves (Table 2). Phytochemical analysis of F. platypylla extracts of chloroform and methanol from leaves showed the presence of steroid, flavonoid and phenols. The leaves on the other hand have additional phytochemical (glycoside) (Table 3). The same trend of phytochemical composition was also obtained in the stem bark extract of the solvents (Table 4) (Figure 2-5).
Figure 1:Pictorial representation of phytochemical test for secondary metabolites:
A. Alkaloids (creamy coloration)
B. Glycoside (dark brown ring formation)
C. Flavonoid (cloudy formation)
D. Phenols
E. Tannin.
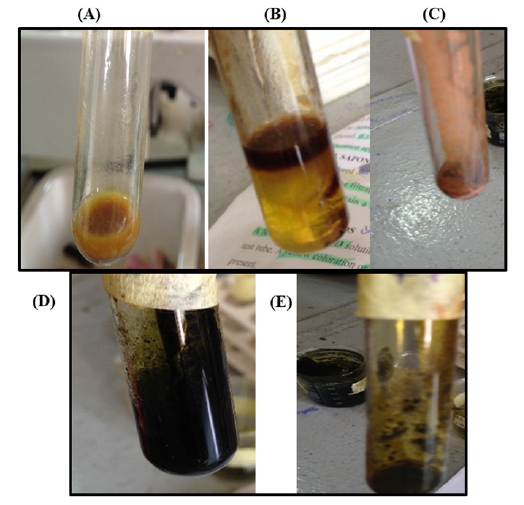
Table 1:Phytochemical analysis of leaf extract of M. indica.

Table 2:Phytochemical analysis of stem bark extract of M. indica.

Table 3:phytochemical analysis of leaves extract of F.platypylla.

Table 4:phytochemical analysis of leaves extract of F.platypylla.

Figure 2:Percentage inhibition (%) of M. indica LEAF and stem back extract (chloroform and methanol) on A. niger.
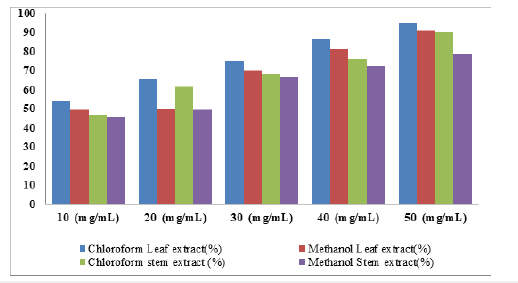
Figure 3:percentage inhibition (%) of M. indica leaf and stem back extracts (chloroform and methanol) on A. flavus.
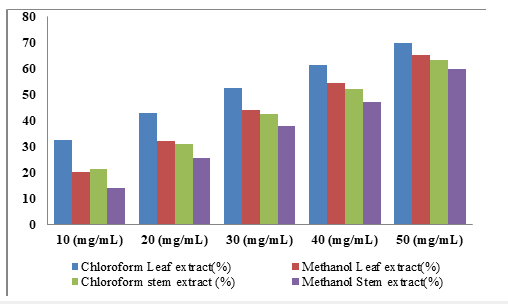
Figure 4:percentage inhibition (%) of F. platyphylla leaf and stem back extracts (chloroform and methanol) on A.niger.
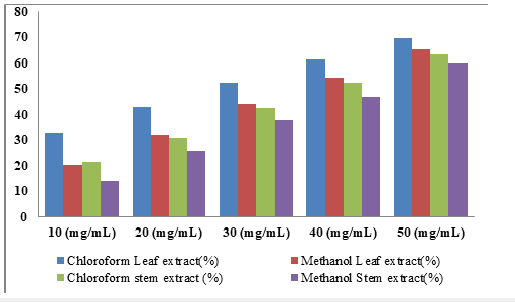
Figure 5:Percentage inhibition (%) of F. platyphyla leaf and stem bark extracts (chloroform and methanol) on A.niger.
Isolation and Identification
During the survey of fungi associated with rot disease of onions in the site, two fungi were isolated and identified as Aspergillus niger and Aspergillus flavus from three samples as presented in Table 1. This survey showed that samples 1, 2 and 3 all have higher number of Aspergillus niger species. However, fewer number of Aspergillus flavus species were found to be present in samples 1 and 3 while sample 2 indicated significant presence of the fungus. Additionally, it was found that all the samples had negative presence of Penicillium spp. and Rhizopus spp.
In vitro efficacy of M. indica on the growth of onion bulb rot fungi
Effect of M. indica leaf and stem bark extracts on the growth of A. niger: The percentage inhibition on the growth of A. niger due to application of different concentrations of M. indica leaf and stem bark extracts were presented in Table 5. At 50mg∕ ml of chloroform leaf extracts, 94.54% inhibition was recorded compared to the methanolic extract with 91.00%. Similarly, the percentage inhibition at other concentrations of chloroform leaf extracts 10 (54.15%), 20 (65.33%), 30 (74.94%) and 40mg/ml (86.08%) also indicated increasing inhibition compared to the methanolic extracts with 10 (49.31%), 20 (50.06%), 30 (69.75%), and 40mg/ ml (80.92%). The percentage inhibition of chloroform stem extracts, 46.91, 61.65, 68.03, 76.09 and 89.79% for 10, 20, 30, 40 and 50mg/ml concentrations respectively also indicates stronger inhibition against A. niger than the corresponding inhibition with methanol extracts with 45.60, 49.60, 66.56, 72.14 and 78.64%. This result showed that leaf extracts from both methanol and chloroform revealed higher inhibition compared to the extracts from stem back at all concentrations (10, 20, 30, 40 and 50mg ∕ml). However, the chloroform extracts from the leaf and stem indicate slightly higher percentage of inhibition than the methanolic extracts from these parts of the plant.
Table 5:The Results of the Organisms Isolated. (++) = High; (+) = Moderate (─) = Absence

Effect of M. indica leaf extract on the growth of A. flavus: The percentage inhibition on the growth of A. flavus due to application of different concentrations of M. indica leaf and stem bark extracts were presented in Table 6. The result showed high inhibition at 50mg∕ml concentration of the extracts with 93.63 and 90.68% in chloroform and methanol extract of leaf respectively but a slightly lower percent inhibition of 89.41% and 79.15% were recorded in chloroform and methanol extracts of stem bark. At 40mg/ml concentration of chloroform and methanol leaf extracts, 85.45 and 82.65% inhibitions were recorded. However, the stem extract showed lower percentage of inhibition with 82.05 and 72.79 for both chloroform and methanolic extracts respectively. This lower trend continued as the concentrations of the extracts were decreased. These include 77.00% (30mg/ml), 70.75% (20mg/ml) and 62.06% (10mg/ml) for chloroform leaf extract and 76.20%, 68.11%, 59.53% for 30, 20 and 10mg/ml concentrations of the leaf extracts from methanol respectively. The percentage inhibition of the stem extracts from the solvents indicates also that at a concentration of 50mg/ml, maximum inhibition of 93.63% and 90.68% against A. flavus were recorded for leaf extracts from chloroform and methanol respectively. Similarly, 89.41%, and 79.15% inhibitions were obtained with 50mg/ml of the stem extracts of chloroform and methanol respectively. In addition, 82.05%, 76.59%, 69.34% and 65.04% inhibition and 72.79%, 67.83%, 59.73% and 51.49% inhibition against A. flavus were recorded for 30, 20, and 10mg/ ml concentrations of stem extracts from chloroform and methanol respectively.
Table 6:Percentage (%) Inhibition of A. niger by extracts from M. indica leaf and stem bark.

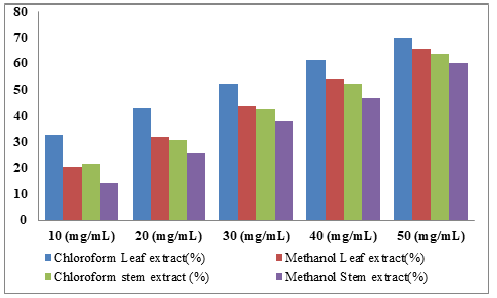
Effect of F. platyphylla leaf and stem bark extracts on the growth of A. niger: The percentage inhibition on the growth of A. niger due to application of different concentration of F. platyphylla leaf and stem bark were presented in Table 7. The result showed high inhibition (77.28%) at 50mg/ml concentration of chloroform extract from leaf and 71.97% for stem bark. Moderate inhibitions were recorded at concentration of 40mg/ml with 58.34% and 54.36% in methanolic extract of leaf and stem bark respectively. This decrease in percentage inhibition continues as the concentration of the extracts in the solvents decrease which is evident for 30mg/ ml concentration with 58.78% and 53.78% in chloroform and methanol leaf extracts respectively. Similarly, 54.25% inhibition against A. niger was recorded for chloroform extract and 49.77% inhibition obtained for methanolic extract of the stem. Additionally, when 20mg/ml and 10mg/ml concentrations of the leaf extracts was tested against the fungus, 56.42% and 44.20% inhibitions; 41.30% and 30.95% were shown in chloroform and methanol leaf extracts respectively. The stem extracts from both solvents also revealed a decline in percentage.
Table 7:Percentage (%) Inhibition of A. flavus by M. indica Leaf and stem bark Extracts.

Effect of F. platyphylla leaf and stem bark extract on the growth of A. flavus: The percentage inhibition on the growth of A. flavus due to application of different concentration of F. platyphylla leaf and stem bark were presented in Table 8. The result showed moderate inhibition (69.71% and 65.31% in chloroform and methanol extract of leaf; 63.41% and 59.92% chloroform and methanol extract of stem bark) at 50mg/ml concentration of the extracts. At 40mg/ml concentrations 61.38% and 54.39% were recorded in chloroform and methanolic leaf extracts; 52.18% and 46.95% recorded in chloroform extract of stem bark. 52.41% and 43.95% inhibition were recorded in chloroform and methanol leaf extracts while 42.54% and 37.92% inhibition were shown for chloroform and methanol stem extracts at 30mg/ml. Lower inhibitory activities were recorded at 10 and 2omg/ml of the extracts as shown in Table 9. The isolates were aseptically inoculated into healthy susceptible onion, the characteristic of the organism originally observed were also noticed again, all the fungus were confirmed as the causative agent of onion rot. The result of pathogenicity tests carried out show that, the organisms were pathogenic and were the actual agent of the spoilage onion bulbs and can also infect others plants like fruits and also many other vegetables.
Table 8:Percentage (%) Inhibition of A. niger by F. platyphylla Leaf and stem bark Extracts.

Table 9:Percentage (%) Inhibition of A. flavus by F. platyphylla Leaf and stem bark Extracts.

Discussion
Onion is a plant of economic importance and widely cultivated in the world [14,15]. It is an important vegetable crop [16,17] whose distinctive flavor is appreciated by people throughout the world and therefore ranks fourth in world production of vegetable with a volume of 64.101 metric tons annually [13]. Onions suffer from many diseases from pre harvest to post harvest period. The survey conducted at the international level revealed that about 35-40% of onions are lost due to damage caused by different diseases [18]. A number of microorganisms are responsible for bulb rotting of onion, but among them, fungi are the main causal agent responsible for pre- and post-harvest period losses in the onion, Currah and proctor [19]. Various species of Aspergillus pathogens are reported to cause blue mold on onion bulbs during storage. The blue molds are frequently isolated from stored diseased bulbs of local cultivars of onion, Hussain et al. [20]. Aspergillus niger is able to produce mycotoxin which reduces the quality and quantity of food products and feedstuff which is a potent hepatic- carcinogen in humans and animals, Paster et al. [21].
The result of this study shows that Aspergillus niger and Aspergillus flavus isolated from rotten onion bulb and were associated with onion bulb rot. The result also reveals that A. niger was responsible for black coloration on onion bulb, as described previously by Adongo [2] that A. niger also causes the black mold diseases causing significant number of postharvest losses in onion all over the world. The present study is in conformity with that of Shehu [22] and KO et al. [23] that also isolated this fungus from rotten onion bulbs and that the storage under ambient conditions in the tropics. The fungi isolated from were confirmed to be pathogenic on onion bulbs and A. niger was highly pathogenic and caused the highest amount of rot on onions bulb in 4-5 days followed by A. flavus. However, Ibrahim [24] and Joon et al. [25] indicated that Aspergillus and Botrytis species were most frequently encountered during isolations in many parts of the tropical and humid regions. The pathogenicity test establishes infection of the onion bulbs through mechanical injuries and openings from animals etc. The effect of plant extracts on fungal growth and disease development may vary depending on the solvent used for extraction and also the concentration of the extracts [26]. Percentage inhibition of M. indica and F. platyphylla against the fungi revealed that chloroform extracts of leaf are more sensitive and effective on the fungi In vitro as it significantly reduces fungal growth by more than 90%. Additionally, all extracts at the highest concentration (50mg/ml) reduces disease incidence associated with the two fungi (A. niger and A. flavus). This is similar for other concentrations even though with reduced percentage inhibition. This by implication means that M. indica and F. platyphylla leaf and stem bark extracts possesses antifungal potential against A. niger and A. flavus, in a similar result reported by William [27], [28-42].
Conclusion
The findings from this study revealed that most of the spoilage of onions in Kano state are caused by these two isolated fungi, A. niger and A. flavus. These fungi were found to be highly pathogenic on onion bulbs. The study further indicated that the plant extracts had fungicidal properties with M. indica extracts having the more inhibitory properties than F. platyphylla extracts with a directly proportional increase in the concentration of the extracts compared with the control. Plant materials are cheaper to obtain and not harmful to lives than the pesticides which are harmful and often costly. Further research is encouraged to confirm the effectiveness of these extracts on the growth of fungi in-vivo.
References
- Ara MAH, Khatun ML, Ashrafuzzaman M (2008) Fungi causing rot in onions at storage and market. Journal of Bangladesh Agricultural University 6(2): 245-251.
- Adongo BA, Kwoseh CK, Moses E (2015) Storage rot fungi and seed-borne pathogens of onion. Journal of Science and Technology 35(2): 13-21.
- Kurmukov AG (2013) Phytochemistry of medicinal plants. Medicinal Plants of Central Asia: Uzbekistan and Kyrgyzstan 1(6): 13–14.
- Ramawat KG, Dass S, Mathur M (2009) The chemical diversity of bioactive molecules and therapeutic potential of medicinal plants. Herbal Drugs: Ethnomedicine to Modern Medicine, pp. 7-32.
- Talba AM, Suleiman MM, Raji MA, Oniye SJ (2014) Phytochemical screening and in-vitro antibacterial activity of Mangifera indica (mango) kernel on Aeromonas caviae. IOSR J Pharm 4(10): 45-50.
- Amos S, Binda L, Chindo B, Akah P, Abdulraman M, et al. (2001) Evaluation of methanolic extract of Ficus platyphylla on gastrointestinal activity. Indian Journal Experimental Biology 39(1): 63-67.
- Abdel Hameed ES (2009) Total phenolic contents and free radical scavenging activity of certain Egyptian Ficus species leaf samples. Food Chemistry 114(4): 1217-1277.
- Abdelnaser AE, Shinkichi T (2010) Preliminary phytochemical investigation on mango leaves. Word J Agri Sci 6(6): 735-739.
- Adesegun SA, Coker HAB (2001) Plants used in traditional medicine against malaria. Nigerian Journal of Pharm 32: 50-62.
- Chindo BA, Amos S, Odutola AA, Vongtau HO, Abbah J, et al. (2003) Gamaniel Central nervous system activity of the methanol extract of Ficus platyphylla stem bark. Journal of Ethnopharmacological 85(1): 131-137.
- Nadembega P, Boussim JI, Nikiema JB, Poli F, Antognoni F (2011) Medicinal plants in Baskoure, Kourittenga province, Burkina Faso: An ethnobotanical study. Journal of Ethnopharmacology 133(2): 378-395.
- Chindo B, Joseph A, Amos S, Lilly MN, Abdullahi HY, et al. (2009) Anticonvulsant properties of saponins from Ficus platyphylla stem bark. Brain Research Bulletin 78(6): 276- 282.
- Robert AS, Ellen SVG (1988) Introduction to food-borne fungi. Pomsen and Looijen B.W. Wageningen.
- FAO (2005) UN Food and Agriculture Organization.
- Awuah RT, Kwoseh C, Kuranteng SL, Okpala ROC, Amoako AI (2009) Appearance of fusarium basal rot disease of onion in the Kwahu sought district of Ghana. Ghana Journal of Horticulture 7: 84-88.
- Sinnadurai S (19970) A note on the bulbing and flowering habit of the bawku onion. Tropical Agriculture Trinidad 47: 77-79.
- Kyofa BM, Blay E, Braum M, Kuehn A (2000) Good agricultural practices and crop protection recommendations for selected vegetables. Handbook of Crop Protection in Ghana 5: 95-108.
- Gupta RP, Verma LR (2002) Problem of diseases during storage in onion and garlic and their strategic management. In implication of plant diseases on produce quality. pp. 55-62.
- Currah L, Proctor FJ (1990) Onion in tropical region. Natural Resources Institute, Chatham, Maritime, Kent, UK, p. 79.
- Hussain FN, Abd Elrazik FA, Darweish A, Rushdi MH (1977) Survey of storage diseases of onion and their incidents in upper Egypt. J Phytopath 9: 15.
- Paster N, Menasherov M, Ravid U, Juven B (1995) Antifungal activity of oregano and thyme essential oils applied as fumigants against fungi attacking stored grain. J Food Protects 58(1): 81-85.
- Shehu K, Muhammad S (2011) Fungi associated with storage rots of onion onion bulbs in Sokoto. Nigeria. International Journal of Modern Biology 1(1): 1-3.
- Ko S, Huang J, Wang J, Subramanyam S, Chang W (2002) Evaluation of onion cultivars for resistance to aspergillus niger, the causal agent of black mold. Journal of American Society of Horticultural Science 127(4): 697-702.
- Ibrahim SD (2005) Fungal pathogens Associated with stored onions in Maiduguri and Bama Towns of Borno state. University of Maiduguri, Nigeria.
- Joon TL, Don WB, Seun HP, Chang KS, Youn SK et al. (2001) Occurrence and biological control of post-harvest decay in onion caused by fungi. Plant Pathology Journal 17(3): 141-148.
- Kalimathu K, Vijayakumar S, Senthikumar R (2010) Anti-microbial activity of the biodiesel plant, Jatropha curcas. Intern J Pharm Bio Sci 1(3): 1- 5.
- William Q (2008) Least toxic control of plant diseases. Botanic garden. Natural Disease Control 11: 225.
- Abduljabar A (2004) Economics of onion retail marketing in MMC Borno state Nigeria. Unpublished B. Agric Project Department of Agricultural Economics and Extension University of Maiduguri Borno State, Nigeria.
- Abdulsalam AA, Zakari BG, Chimbekujwo IB, Channya FK, Bristone B (2015) Isolation and control of fungi associated with neck rot disease of onions (Allium Cepa L.) In Bama, Borno State, Nigeria. Global Journal of Biology, Agriculture & Health Sciences 4(4): 35-39.
- Agrios GN (2005) Plant pathology. (5th edn), London, UK, pp. 330-340.
- Agricultural Extension Research and Liaison Services Report (1985).
- Baiyewu RA, Amusa NA, Ayoola OA, Babalola OO (2007) Survey of the post-harvest diseases and aflatoxin contamination of marketed pawpaw fruit (Carica papaya L.). Afr J Agric Res 2(4): 178-181.
- Balali M, Malakouti MJ (2002) Comparison of various methods of identifying trace elements and magnesium sulphate in wetland wheat? in different provinces of Iran. Agriculture Education Publications, pp. 135-152.
- Bamgbose T (2012) Economic importance of fungi in agriculture. p. 13.
- Chilvers MI, Dutiot LJ (2006) Detection and identification of botrytis species associated with neck rot, scape blight and umbel blight of onion. Plant Health Progress 7(1).
- Chukwuka KS, Okonko IO, Adekunle AA (2010) Microbial ecology of organisms causing pawpaw (Carica papaya L.) fruit decay in Oyo state, Nigeria. Am Eurasian J Topical Sci 2(1): 43-50.
- Gomez KA. Gomez AA (1984) Statistical procedures for agricultural research. (2nd edn), General & Introductory Agriculture. John Wiley and Sons, p. 704.
- Grubben GJH, Denton OA (2004) Plant resources of tropical Africa 2. Vegetables. PROTA foundation, Wageningen, Netherlands, Wageningen.
- Joseph B, Raj SJ (2010) Phytopharmacological and phytochemical properties of three Ficus species. Int J Pharma Bio Sci 1: 246-53.
- Raju K, Naik MK (2007) Survey and assessment for the post-harvest diseases of onion in North-Eastern Karnataka. Karnataka Journal of Agriculture Science 20(1): 164-165.
- Salisu LH, Aliyu MS, Zaharau UI (2019) Storage performance of modern onion storage structure facility Makani model. International Journal of Agriculture, Environment and Bioresearch, pp. 136-137.
- White K, Zellner J (2008) Scientific classification and etymology historical origins. Seminar 235, Food for Thought.
© 2024 Sunusi Bataiya Buhari. This is an open access article distributed under the terms of the Creative Commons Attribution License , which permits unrestricted use, distribution, and build upon your work non-commercially.
 a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in
a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in 







.jpg)






























 Editorial Board Registrations
Editorial Board Registrations Submit your Article
Submit your Article Refer a Friend
Refer a Friend Advertise With Us
Advertise With Us
.jpg)






.jpg)














.bmp)
.jpg)
.png)
.jpg)










.jpg)






.png)

.png)



.png)






