- Submissions

Full Text
Journal of Biotechnology & Bioresearch
Energy Supply, Climate Change and Carbon Cycle–What Would Bring the Use of Recent Carbon?
Manfred Ringpfeil*
Kurfürstenstraße 104, 10787 Berlin, Germany
*Corresponding author:Manfred Ringpfeil, Kurfürstenstraße 104, 10787 Berlin, Germany
Submission: November 27, 2023;Published: December 15, 2023

Volume5 Issue1December 15, 2023
Abstract
Climate change is to be eliminated through the annual transfer of a defined quantity of non-agriculturally produced plant biomass from the earth’s aerobic natural carbon cycle to the anaerobic lithosphere, which is not covered by this cycle. This amount of biomass should correspond to the amount of fossil-based CO2 that is released into the atmosphere from industry in the same time. The further use of fossil carbon, in particular for energy production, would thus be possible without any negative consequences for the climate. The biomass stored in the lithosphere shall be subjected to biological and chemical reactions that produce hydrocarbons analogous to those of natural gas and mineral oil. As a result, the current time limit on the availability of these raw materials could be lifted. Introducing global control of the processes involved is useful in order to maintain the constancy of the atmospheric CO2-concentration. Problems to be overcome are to provide the transport capacity for collecting the required amount of biomass above ground and to convey it underground. Furthermore, the spaces in the subsoil must be determined that are suitable for the storage of the biomass and the formation of the hydrocarbons. The proclaimed decarbonization should be redefined in terms of content and goal.
Keywords:Biogas; Biomass ; Biotechnology ; Cannizzaro reaction (Specific Redox Reaction); Carbon cycle; Climate change; CH4 (Methane); CO2 (Carbon Dioxide); Decarbonization; Fossil carbon; Green energy reserves (Wild Vegetation); Hydrocarbons
Introduction
Energy is not in deficit for humans on earth. A whole range of primary energies are present: Solar radiation, gravity, nuclear energy, geothermal energy, fossil carbon as coal, mineral oil and natural gas, recent carbon as biomass of plants, animals and microorganisms, water currents, wind, ocean waves, tides and static electricity. The development and use of fossil carbon to provide energy and materials has provided mankind with a technical basis that promotes prosperity, but at the same time has also triggered nature destroying climate change. The latter must be switched off [1]. Would it be possible to neutralize the influence of fossil carbon use on the climate when further using it or when switching production of usable energy from fossil to recent carbon?
Recent carbon is the carbon stored in our planet’s atmosphere as CO2. It consists of two components with identical properties but different origins: Original rCO2*), the carbon of which was always part of the natural carbon cycle, and fossil-derived fCO2**), the carbon of which in prehistoric times happened to get into the lithosphere as biomass [rCH2O]***), converted there into coal [fC], mineral oil [fCH2] and natural gas (fCH4), deposited there and thereby withdrew from the natural carbon cycle. Atmospheric rCO2 remained virtually free of fCO2 for a long time, until the Industrial Revolution initiated the mass consumption of the fossil carbon stocks from the lithosphere, as a result of which the fossil component of the r+fCO2****) mixture in the atmosphere increased year by year. The carbon mixture of the current r+fCO2 amounted to 880 Gt5*) C in 2018 [2], corresponding to 3200 Gt CO2. Currently, 37 Gt fCO2 are added every year by the fossil carbon using industry [3]. The fossil component causes the recent r+fCO2 mixture in the atmosphere to increase. As a result, the supply and demand for CO2 in nature is in contradiction. The inflow of fCO2 and r+fCO2 outweighs the outflow of r+fCO2 from the atmosphere. What needs to be done to get it back into balance? Not only reduce the inflow, but also increase the outflow!
Recent carbon, r+fC in r+fCO2, is a component of all living beings and thus also the material basis of today’s natural carbon cycle [4]. Today’s living beings including humans, all consist of a mixture of carbon, which comes on one hand from nature, rCsup> and on the other hand from the fossil carbon processing industry, fC, that is the mixture r+f. The same properties of both carbons mean that this has no consequences for life. But it has for the atmosphere. It is being overloaded by fCO2 introduced via industry and triggering disturbances in the outflow of infrared heat from earth into space, leading to present climate change. In this respect, fossil carbon, fC, has an undesirable impact on life. A creeping nature-destroying function must be added to its prosperity-bringing function. It expresses itself more and more visibly in threatening natural events. The answer of the leaders of human society is decarbonization [5], banish the carbon! It has never been clearly stated what the carbon should be banned from. Out of life? Out of electric power generation? Out of economy? Decarbonization of life is not possible because life’s chemical basis is carbon. Decarbonization of electric power generation has already progressed, but is increasingly proving to be insufficient. So probably decarbonization of economy? In practical life one designates such a measure that is taken overhastily, also as a “quick shot”. This is what happened with regard to decarbonization with consequences. It hit the negative as well as the positive side of carbon use. Apparently, it was only intended to reduce the flow of CO2 into the atmosphere. However, there have been inflationary phenomena in more or less all countries, significant price increases for everyday necessities, including energy in particular, some adventurous variants of securing national energy supplies and also disbelief, indifference and even resistance to measures to reduce CO2 amount in useful energy production and thus to general uncertainty about the future of energy supply. Nonetheless, decarbonization has its place in the overall effort to find solutions against climate change. Because it is becoming apparent that climate change cannot be switched off with a single method alone.
The climate change currently unfolding in our world has come about because of an imbalance in the flow of CO2 into and out of the atmosphere
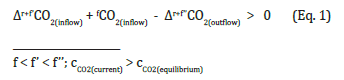
When inflows are greater than outflows, the concentration of CO2 in the atmosphere, cCO2 increases; it is getting warmer. If the outflow is greater than the inflow, cCO2 falls; it’s getting colder. Corrections for equality can be made via both inflow and outflow. The arsenal of available methods is just beginning to be put together. A coordinated international approach has not yet been brought about. It is clear that climate change is a global problem due to its trigger, fCO2, in the atmosphere. It can only be solved globally. Forcing ahead with a solution will not benefit the initiators if others continue to release their fCO2 into the atmosphere. It is now a question of not allowing cCO2 to continue to rise [6]. Immediate solutions seem to be necessary, therefore. However, basic questions such as clarifying the connections between the occurrence of fCO2 and its effects on the natural carbon cycle should also be addressed in order to find effective solutions.
The natural carbon cycle is, together with the use of recent carbon, of basic importance for the elimination of climate change. The carbon cycle works at the surface of the earth, extends across the biosphere (including the hydrosphere and atmosphere). It allows plants and phototrophic microorganisms to absorb CO2, uses sunlight and water to reduce the absorbed CO2 to carbohydrate, [CH2O], builds this into the biomass of the plant or phototrophic microorganism, spreads, mainly through uptake of plants by (nonphototrophic) microorganisms, animals and humans and provides the energy for their life manifestations as well as those of the plants and phototrophic microorganisms through the oxidation of the carbohydrate and the resulting further semi-oxidized carbon compounds to the final oxidation product, CO2, flowing back into the atmosphere. When the life of organism ends, in contact with the atmosphere, oxidation processes occur and their biomass turns back into CO2 and some non-carbon substances. In effect the gaseous CO2 can be stored temporarily in solid form as biomass in the plant and the other organisms, thus withdrawn from the atmosphere (Figure 1).
Figure 1:Natural Carbon Cycle (NCC)*).
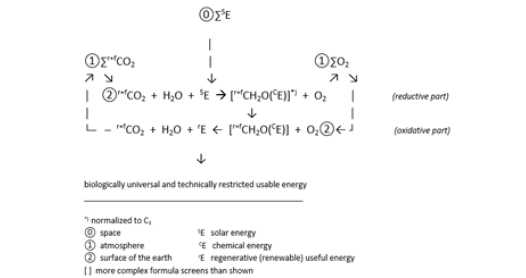
If part of this biomass is separated from the carbon cycle and placed in the lithosphere, it cannot be converted into CO2. The fCO2 from the industry can now take this place. The balance between CO2 inflow and outflow is maintained and cCO2 remains constant at the equilibrium level (Figure 2). Unlike the CO2, both the carbohydrate and the biomass contain chemical energy that can be passed on to other organisms, e.g. as food or feed. However, in today’s machine park, biomass can only be used to a limited extent, i.e. selectively, as raw and heating material or fuel for the production of other carbonic substances, heat or work. In all of these processes, CO2 reappears, this time as a material by-product of the volatile energy. The useful energy from this circuit is of the form and strength necessary to allow biological processes, including human physical life, to take place in this circuit. However, it has neither the form nor the strength to be suitable for machines in the modern sense. However, useful energy that can be obtained thermally from fossil carbon has the form and the strength to be suitable for machines. In 2019, at least 80% of the total energy used to drive technical processes came from fossil carbon [7]. On the other hand, it is not suitable for driving biological processes
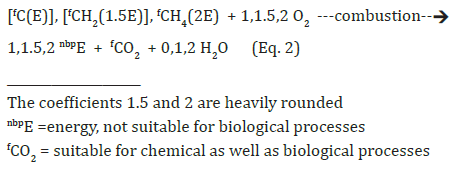
Figure 2:Carbon Cycle Modified Negatively (+fCO2) and Positively (-r+fCO2) by Technical Measures (mNCC)*).
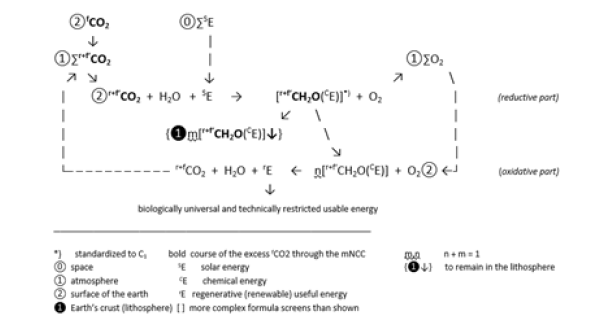
As already mentioned, the fossil carbon raw materials used for these technical processes currently release annually 37 Gt fCO2 into the atmosphere [3] and thus increase the r+fCO2 quantity of 3200 Gt there by more than 1% yearly. It is assumed that the carbon cycle reacts to this increased supply of raw materials by allowing increased biomass formation in or with the plants and phototrophic microorganisms in its reductive part. However, the processes in the oxidative part of the carbon cycle break down this biomass back into CO2 at the end of the growth or life of the organisms. As a result, there is only a build-up of CO2 in the atmosphere with the result of climate change. What are the ways out? The decarbonization route relies on the generation of electrical energy from wind and sunlight and, more recently, geothermal energy. But electricity as useful energy only succeeded because the carbon raw materials took over the transport over long distances as well as the storage of large quantities. A tandem way of working had developed with the transport of the carbon raw materials to the point where their chemical energy was converted into electrical energy needed there. This model can be retained for recent carbon commodities. However, this doesn’t work with the source of recent carbon, the CO2 of the atmosphere. This molecule does carry the carbon, but no chemical energy. It has to be charged to the carbon of the CO2 by means of chemical reduction. This is undoubtedly a costly process.
It requires the use of two hydrogen molecules to tear two oxygen atoms from the carbon of the CO2 to make room for two or four hydrogen atoms
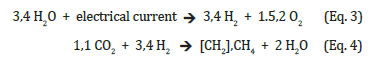
Nature does the same thing using free solar energy and some technical aid in two steps, but in a completely different way: Without the use of electricity for hydrogen production, i.e. without the use of commercial amounts of hydrogen itself, but with a large area requirement for capturing sun light to reduce CO2 using water in plants and phototrophic microorganisms for their production of biomass and oxygen. From this there is still a need to transfer the biomass to necessarily punctiform anaerobic processing places in order to obtain machine-suitable energy in form of hydrocarbons.
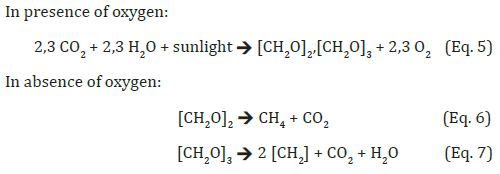
The real and assumed formation equations suggest a comparison with the Cannizzaro reaction [8]. The fundamental importance of this reaction for the formation of hydrocarbons as raw materials for useful energy production is suggested by the fact that results in a concentration of energy in the material product by separating off energy-less or energy-poor parts of the original molecule. It is only through this type of concentration that the energy from the biomass becomes suitable for machines. The fact that the wild vegetation on earth represents a significant energy potential speaks for the pursuit of energy production with this biological variant via the plant. Formally, it corresponds to at least a hundred times the world’s annual production of electricity [9,10]. Other reasons are that an optimal condition of the surface of the earth for the growth of plants can be more easily maintained when it is covered with them and that the current agriculture does not have to be called upon for this. The required biomass can be taken from the “green energy reserves” on the surface of the earth. So far, however, neither of the two variants has promised success in mitigating climate change by reducing the concentration of CO2 in the atmosphere, cCO2. On the contrary, cCO2 has continued to rise [3]. The chemical variant is not able to counteract this, because the technical prerequisites are not remotely available to reduce cCO2. However, even the biological variant, which initially requires nothing other than the presence of living and growing plant biomass, has so far not been able to eradicate the excess CO2 from the atmosphere caused by fCO2 - not even the entire plant growth on earth. Plant growth, with its global formation of 450 Gt biomass-C/a [10], should actually be able to absorb the 10 Gt fCO2-C/a brought in by industry (corresponds to 37 Gt fCO2/a [3]). Surely, that will happen. But most plants on earth are annual plants that follow their seasonal rhythm and are therefore subject to oxidative processes at the end of their growth or life phase, which turn them back into CO2, which in turn flows into the atmosphere. The considerable amount of energy built up in them, which exceeds that of technical services such as electricity generation or carbon production by orders of magnitude, is dissipated unused as heat into the environment. For comparison, world agriculture currently produces 13Gt of biomass- C/a [11] in the form of products of this biomass. This corresponds to 48 Gt CO2/a that is removed from the atmosphere. But even from this a considerable part of the carbon, surely used to sustain life as food or feed, ultimately returns to the atmosphere as CO2. Quantitatively, the following picture emerges:
Equilibrium between atmospheric CO2 and terrestrial plant biomass is
CO2 : plant biomass [CH2O] = 880 : 450 Gt C (Eq. 8)
So the task is to remove 37 Gt r+fCO2/a = 10 Gt r+fC/a from the atmosphere to make room for the same amount of fCO2 from industry. This would mean that the goal of stopping climate change would be satisfied by the expected stability of the current CO2 concentration in the atmosphere, cCO2, and would even ensure the continued existence of a carbon processing industry. This idea was already advocated by MIT at the beginning of the awareness that the fCO2 released into the atmosphere would cause damage to nature [12]. The fCO2 should be stored in the lithosphere. However, this groundbreaking solution met with resistance from the population, especially in Germany. People justifiably did not want any suffocating gas to be present under pressure under their homes [13]. The realization of this idea was discontinued, but continued in attempts [14], so far without response. Recently, the question of storing fCO2 under the North Sea is raised [15].
Figure 3 names the trigger quantities of coal as carbon, mineral oil as [fCH2] and natural gas as fCH4, in amounts, as each of them alone would produce the amount of fCO2 which is released every year by the carbon processing industry into the atmosphere. Further, Figure 3 names the amount of wet biomass which must be removed as countermeasure from the carbon cycle in order to switch off climate change.
Figure 3:Climate Change Drivers (Triggers) and Countermeasure
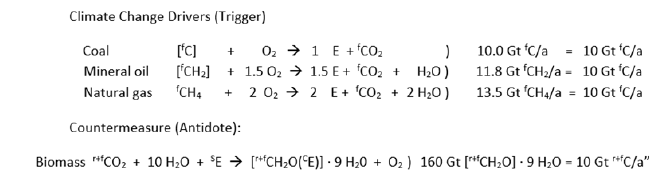
The refusal of CO2 storage led to the idea of introducing the natural derivative of CO2, plant biomass, [CH2O] into the lithosphere instead of the CO2 itself. The similarity to the prehistoric, natural and random transfer of biomass into the lithosphere, resulting in the formation of coal and hydrocarbons of mineral oil and natural gas, is obvious(although coal obviously followed a fundamentally different formation mechanism than mineral oil and natural gas). What happened did not affect humanity because it did not exist at that time. The storage of biomass in the lithosphere today must take into account humanity’s interests in the lithosphere and bring the temporal and quantitative changes and movements of biomass into scales that can be managed by humans and their current technology.
Solution I
Bring biomass into the lithosphere and store it there. Based on the 37 Gt fCO2/a, currently produced industrially as a by-product of useful energy [3], the equivalent of 25 Gt dry biomass/a will be used as a calculation basis. However, biomass as an organismic energy carrier can only be obtained in the required quantities as wet plant biomass. The 25 Gt dry biomass/a correspond to 160 Gt wet biomass/a. This amount is certainly present, because the estimated 450Gt of plant carbon/a in the current world [10] means the presence of 7200 Gt wet biomass/a. Agriculture currently moves and processes 13 Gt plant carbon/a [11] corresponding to 210 Gt wet biomass/a. Compensating for 37 Gt fCO2/a added to the atmosphere would require the movement and storage of 160 Gt wet biomass/a away from the atmosphere (Figure 4).
Figure 4:Requirements for movement of wet biomass to balance industrial production of fCO2 in comparison to movements of wet biomass in agriculture.

The movement of the wet biomass for fCO2 compensation is therefore within the realm of human possibility. A certain selection of standard types is even conceivable. Preference should be given to those on which there is a high density of settlement with wild vegetation. However, the choice of location must also take into account that the underground storage options for the biomass should be as close as possible to the preferred aboveground production areas. Furthermore has to be regarded that not every underground location will be suitable for storing biomass. Otherwise, it should be considered to use the high binding of water in the biomass for its enzymatic liquefaction [16] and thus to facilitate its transport. An energy-saving solution for transport both for the collection of wild vegetation and for its underground storage is of significant importance for the application of this kind of eliminating climate change. Therefore, any other method would be welcome that reduces the amount of biomass to be moved in order to eliminate climate change, e.g. as the generation of electrical energy through wind and sun or the undersea storage of CO2.
The question is whether this solution of storing biomass in the lithosphere has already exhausted the possibilities for curbing climate change? Basically, it is just a variant of the CCS solution, Carbon Capture and Storage [12,14]. Efforts have now long been directed towards finding CCU solutions, Carbon Capture and Utilization [17]. The stored biomass contains energy. Chemical as well as microbial reactions can occur voluntarily or under the influence of geothermal heat. This must be taken into account during storage in view of the spread of undesirable substances in the lithosphere. Can these reactions also be used to recover valuable materials during storage? That would mean developing the lithosphere not only as a storage site but also as a production site. However, the biomass converted into the lithosphere according to Figure 2 has become already compensation for inflow of fCO2. If r+fCO2 from this biomass returns to the atmosphere, it must act like fCO2 and increases cCO2. The hydrocarbons resulting from such a reaction also produce fossil-adequate r+fCO2 when burned. In contrast, the CO2 resulting from this biomass, which does not reach the earth’s surface and remains diffuse in the lithosphere, can be viewed as mitigating climate change. Free storage of biomass in the lithosphere, which has not been offset against the supply of fCO2, enjoys greater degrees of freedom. When implemented according to Cannizzaro, both the r+fCO2 resulting from the reaction and from the combustion of the hydrocarbon formed are climate-neutral. However, the process of collecting, transporting and storing wet biomass makes costs, so the reactions that take place in the underground voluntarily or under the influence of geothermal heat should not be left unused.
Solution II
Bring biomass into the lithosphere, let it react to form gaseous and possibly liquid hydrocarbons and prepare them for use or storage. The biogas reaction [18,19], which has been used in agriculture in many ways and has long been used on small scale, can also be interpreted as a Cannizzaro reaction [8]. This splits a semi-oxidized, i.e. energy-containing organic molecule into two molecules through internal charge shifts, one of which contains more or even all of the hydrogen atoms of the starting molecule, the other containing more or even all of the oxygen atoms. Formally, the sum equation applies to the biogas reaction
(CH2O)6 🡲 3 CH4 + 3 CO2 (biologically performed) (Eq. 9)
Its true course was clarified by Thauer [20]. Several types of microorganisms are involved in forming that product pair, CH4 and CO2, which is typical for a redox-reaction. The microbial variant is more complex. Several reactions take place, including decarboxylation of acetic acid. The sum equation is only approximately valid, therefore. Nevertheless, it is not unreasonable to assume that the prehistoric formation of today’s natural gas reserves in the lithosphere took place in a similar way. It is justified to assume that a similar mechanism also applies to the prehistoric formation of the hydrocarbons of mineral oil from carbohydrates.
Normed to C1 that reaction could have been followed
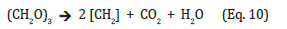
However, chemistry as well as biochemistry knows a simple natural way to produce hydrocarbons from organic acids available in plant and other biomasses, too
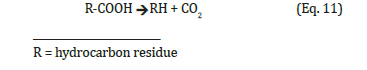
Decarboxylation has apparently played little role in discussions about the formation of mineral oil to date. It is possible that dealing with the question of formation of oil is hindered by the generally accepted opinion that the hydrocarbons of mineral oil would have been formed over long periods of time under high pressure and extreme temperatures? This view should be examined. Process velocities can be divided into mass transfer and reaction velocities and the influence of random geological movements on them could have created the impression of long reaction times although there were only long waits due to mass transfer and normal velocities of reaction. Couldn’t it be that the reaction velocities at prehistoric times didn’t differ at all from those of today? However, a bio-oil production of higher hydrocarbons, similar to that of biogas has not yet been explicitly reported. They must have existed because of the existence of the mineral oil reserves present in the lithosphere. Or could these hydrocarbons also have been produced from the biological material in a purely chemical way? Decarboxylation of organic acids are both biologically and chemically known and may well have found sufficient conditions in the lithosphere for their natural progression. They can also be interpreted as Cannizzaro reactions. However, there is a lack of experimental evidence for this type of formation of oil or higher hydrocarbons from biomass.
It should be taken into account that higher hydrocarbons were not noticed in the aqueous fermentation solutions of the biogas reactions [21], which could give rise to the assumption that they were formed in parallel with methane and homologous gaseous hydrocarbons.
Neo-synthesis of natural gas analogues [22] can be undertaken today. Neo-syntheses of mineral oil analogues can be perhaps already too [23]? If biomass is to be stored in the lithosphere for the purpose of switching off climate change, neo-synthesis of climateneutral hydrocarbons based on Cannizzaro reactions could be used. This would perhaps provide increased benefits compared to simply removing CO2 from the atmosphere or removing biomass from the carbon cycle. But not double benefit. Because, with introducing fCO2 in the carbon cycle and outsourcing the product of CO2, the biomass, the possibilities for mitigating climate change would already be exhausted. But the production of hydrocarbons and their storage in the lithosphere in anticipation of later use would make sense, as would the free storage of biomass for producing hydrocarbons. (These different goals may give reasons for introducing an international authority that would have the task of monitoring and controlling cCO2 as a variable that helps regulating the climate [24].)
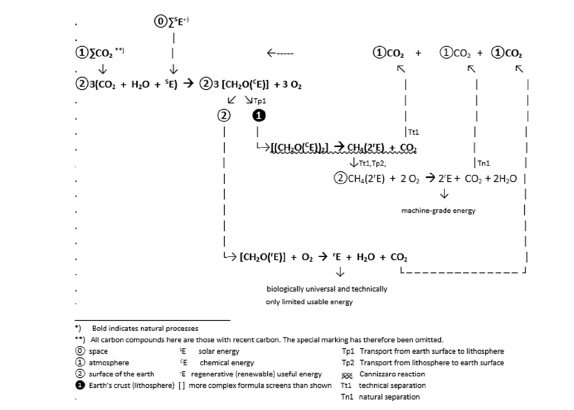
A carbon cycle expanded in this way requires the incorporation of two technical transport processes (Tp1 and Tp2 ), a Cannizzaro reaction (wavy underlined) and two separations of substances, one technical (Tt1) and one natural (Tn1) (Figure 5). In the example above, the proportion of carbon removed from the carbon cycle is arbitrarily set at 2/3. Other relationships might be chosen according to needs of maintaining life and eliminating climate change. The amount of provision of technical energy from the green reserves on earth depends on this relation. If there are no residues left (all hydrocarbons are used via oxidation)
Figure 5:Spatially (lithosphere) and materially (hydrocarbons) expanded carbon cycle (eCC)*).
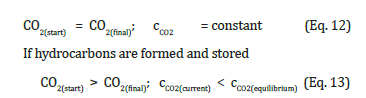
Present climate change is mitigating. This also makes it clear that cCO2 can be used as a benchmark for steering climate movements and in particular for eliminating present climate change.
Discussion
From a human perspective, nature on our planet appears actually more of a giant device for destroying energy than for creating value. It is powered by the carbon cycle. 450 Gt of carbon in 1,650 Gt of energy-free CO2 are converted annually using solar energy and water into 450 Gt of carbon in 1,125 Gt of energy-containing plant biomass [CH2O] and converted back into energyless CO2 using atmospheric oxygen. Most of that biomass energy dissipates into the environment as heat. Only part of the biomass energy goes into maintaining plant, animal, microbial and human life.
Presently, agriculture makes use of 13 Gt biomass-carbon/a [11], equivalent to 210 Gt wet biomass/a and has available a total amount of 450 Gt biomass- carbon/a [10], equivalent to 7200 Gt wet biomass/a grown as plants yearly by nature. Is this difference the “sleeping giant” in human society’s energy economy?
Would it be possible that biotechnological solutions for switching off climate change become the exercise for future production of usable energy for machinery? Can Biology, Chemistry and Physics shift the low energy density of plant biomass to suitable products for use in technology? Can be created methods to collect, store and transform low-grade energy located in yearly renewed plants?
Today, influences on the climate are essentially caused by the warming of the surface of our planet due to the excess CO2 in the atmosphere. Does this warming lead to an accumulation of methane in the atmosphere and thus to a change from CO2-related climate change to CH4sub>-related one as a result of the release of methane into the atmosphere out of the melting permafrost soil? There is no such strong absorption mechanism for CH4 in the atmosphere as there is for CO2. So far there is no other solution than to stop CO2-related global warming timely. Or is it already too late for that? The IPCC has made its warnings clear [25]. The response from the leaders on this planet does not reflect the seriousness of the situation. One can of course hope for a kind of saturation of the CO2-related heat retention on the earth’s surface, but that is not certain. It looks more like a phantom. There is concrete evidence about stopping CO2-related climate change as the solution to avoid a CH4-related climate change continuation [26]. Surely, that will be connected with major expenses. They should be taken into account (The child should not be dropped into the well just to convince that it is not advisable to let it fall in).
Through the material and spatial expansion of the carbon cycle, it is possible to generate the continuous, technically supported natural production of hydrocarbons and use them directly for human activities and indirectly for the conservation and use of nature. This opens up an area of human activity that taps into and directs natural forces. It is essentially designed using biotechnology. The starting point is the CO2, which is converted into a sum of natural meso-oxidized organic compounds (among them carbohydrates and organic acids), from which are formed pairs of completely (hydrocarbons) as well as incompletely (e.g. alcohols) reduced organic substances at one side and completely oxidized carbon (CO2) at the other with the effect to concentrate the energy in the reduced molecules. This opens up further possibilities for transferring energy into technical systems. Simultaneously, hydrocarbons are the best substances for storing CO2 (Figure 6).
Figure 6:Storing Properties of Climate-Active Components of the Carbon Cycle.
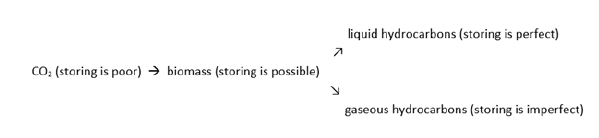
Production of hydrocarbons is bound to production of CO2 at 50, 33, <33% of the carbon of the biomass (see Eq’s 9,10,11). Gaseous hydrocarbons are well storable when the geological conditions allow their perfect isolation from the atmosphere. The production of liquid hydrocarbons needs elucidation to ensure a synthesis of bio-oil, neo-mineral oil. The spectrum of possibilities to be searched includes both purely chemical as well as microbial synthesis routes. The decarboxylation of organic acids and deamination of amino acids can be starting points for the chemical formation of hydrocarbons from biomass. Chemical decarboxylations require little energy as do biological decarboxylation. It seems to be contradictory so far that no formation of liquid hydrocarbons has been noticed in the biogas process. Which competitions or hindrances are subject to preventing the appearance of higher hydrocarbons in biogas fermentations? Is it just the temperature limits for the microbial formation of methane (up to 65 °C) and the chemical formation of higher hydrocarbons (from 100°C upwards)? A sufficient clarification of the prehistoric appearance seems to be helpful for a synthesis of mineral oil analogues today.
In order not to go astray a climate-stable carbon-based economy requires a global monitoring system of the relevant carbon movements in the atmosphere, biosphere, hydrosphere and lithosphere. One of the most important variables to be controlled is the concentration of r+fCO2 in the atmosphere cCO2. It is obviously sensitive to the upside and downside. That means that both the procurement of biogenic energy for use and the avoidance of physical damage to nature are subject to the reference variable cCO2 in the atmosphere. It is therefore necessary to define a range of optimal values of cCO2 in the atmosphere, both downwards in order not to hinder the transfer of CO2 into the plants or microorganisms, and upwards in order not to allow excessive retention of infrared heat on earth. The temperature targets issued [6] are a concrete step towards this. However, it still remains open which is the cheapest way to procure the large quantities of biomass that are to be stored in the lithosphere. Is perhaps the use of the earth’s water-covered areas (that is 71% of the total surface [27]) cheaper than the collection of the wild vegetation on the continents? Economic forms, technologies and equipment are not available for both and would have to develop for both. The water variant holds promise, leading to the colonization of the seas, but leaves open the disruption of marine balances. The mainland variant probably excludes the disruption of natural balances. The current productivity of the sea surface in terms of photosynthesis appears meager [27]. Can it be damaged by the spreading of rootless green plants or the colonization of algae?
Result
Supply of humanity with biogenic energy sources is self-evident and undisputed for its biological reproduction. It is realized by the products of agriculture produced from recent carbon. The technical intrusion into the carbon cycle results in a great responsibility for securing the supply of the material and energetic necessities for life on earth. Extension of the carbon cycle on the lithosphere and there to production of hydrocarbons is the necessary intermediate step between organism-compatible and machine-suitable energy; both being climate-neutral. Climate neutrality of the machinesuitable energy supply is the deciding difference to the presently applied system of energy supply. All systems of non-carbon based energy supply are welcome to relieve the basic climate-regulating carbon system if this will come in use. Quantity-related limits of the carbon-related raw materials and energy are given by the sun irradiation on earth and the demand for biological energy for both mankind and nature.
But whether carbon can be dispensed with, as has now begun with the decarbonization of the economy, should be thoroughly investigated. Ultimately, this is less about economic competition in the use of primary energies and raw materials but more about maintaining the conditions for the earth’s habitability [1]. However, in order to give face to the competitive idea: The aim of the primarily biotechnological variant of eliminating climate change in the course of energy supply as presented here is to increase the prosperity of human society while strictly preserving nature through the use of solar energy via plants, their growth and transport into the lithosphere to store and/or concentrate their energy in climateneutral, reduced-mass carbonaceous carriers. The function of these carbon compounds is multiple: (1) exchange agent against fossil energy sources, (2) input material in the event of future increased energy requirements and (3) reserve material for passing on to future generations.
Conclusion
With the introduction of a carbon cycle economy across four earth spheres – atmosphere, biosphere, hydrosphere, lithosphere – supported by global and locally based CO2 monitoring, an optimal CO2 concentration could be achieved in the atmosphere both for the growth of plants in the biosphere and against the occurrence of CO2-related climate change and can be maintained permanently. Every country on earth could participate according to its ability to dispose of the atmosphere, biosphere, hydrosphere and lithosphere. Experiments based on these options could be undertaken to assess the prospects of implementation. Impacts on the fuel market would be expected, and expansions in the transport industry and mechanical engineering would be necessary. New tasks arise for mining. Colonization and settlement of the seas would be opened.
For science, it could be asked whether it is appropriate to give the same importance and therefore more attention to reduction in nature. In the atmosphere, CO2 is present in a much lower concentration than oxygen (0,04% compared to 21% [27]). However, CO2 is no less important for the existence of human life on earth than oxygen. The design of the expanded carbon cycle enables overcoming the present limitations of renewing the carbon reserves as well as the further use of the available fossil carbon.
References
- https://de.wikipedia.org/wiki/Forschungsgeschichte_des_Klimawandels
- Statista Research Department.
- Ringpfeil M (2018) climate stability and fossil carbon-based energy production-unbreakable opposites? Theses for an attempt to systematically describe the science of overcoming climate change. Industrial Biotechnology 4(4): 178-189.
- Dümpelmann M, Perner J, Westphal B (2016) Decarbonization is a challenge for the industrial nation of Germany. FES Manager Circle Impulses, pp. 51-54.
- https://www.deutscheumweltstiftung.de/unter2grad/?gclid=EAIaIQobChMI1bLD2O6FgAMVsqBoCR1QiggeEAAYAyAAEgKi_vD_BwE.
- https://de.wikipedia.org/wiki/Fossile_Energie.
- Karrer P (1950) Organic chemistry. Elsevier Pub. Co, pp. 188-989.
- https://de.wikipedia.org/wiki/Elektrizit%C3%A4t/Tabellen_und_Grafiken
- https://www.pflanzenforschung.de/de/pflanzenwissen/journal/80-prozent-der-irdischenbiomasse-besteht-aus-pflanzen-10936.
- https://www.umweltbundesamt.de/sites/default/files/medien/479/publikationen/globale_landflaechen_biomasse_bf_klein.pdf.
- (1996) Sleipner fact sheet: Carbon dioxide capture and storage project.
- Archive Lausitzer Rundschau, keywords Vattenfall, CCS technology.
- https://sequestration.mit.edu.
- CO2 storage under the North Sea: Opportunities and risks.
- https://www.biopract-abt.de/en/products/methapract/methapract-cs.
- https://www.iea.org/energy-system/carbon-capture-utilisation-and-storage/co2-capture-and-. utilisation.
- https://www.fnr.de/fileadmin/allgemein/pdf/broschueren/Leitfaden_Biogas_web_V01.pdf
- https://www.ktbl.de/themen/biogas-in-der-landwirtschaft
- https://www.mpg.de/7735111/mpi_fuer_terrestrische_mikrobiologie_jb_2013
- Gerhard Z, Erwin P, Johann H (2022) Quality of waste from biogas plants.
- Ringpfeil M (2021) Neo-gas and neo-oil from biomass feasible goals for continuing carbon-based energy provision without influencing climate EUBCE 2021 conference proceedings 5BV.5.13.
- https://www.deutschlandfunk.de/diesel-aus-dem-druckkessel-100.html
- https://www.umweltbundesamt.de/gaw#global-atmosphere-watch
- https://www.de-ipcc.de/256.php
- https://de.statista.com/statistik/daten/studie/1109076/umfrage/verteilung-von-land-undwasser-auf-der-erdoberflaeche
- https://www.bmbf.de/bmbf/shareddocs/kurzmeldungen/de/2021/07_08/ozean-als-co2. speicher.html
- https://de.wikipedia.org>wiki>Erdatmosphäre/
© 2024 Manfred Ringpfeil. This is an open access article distributed under the terms of the Creative Commons Attribution License , which permits unrestricted use, distribution, and build upon your work non-commercially.
 a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in
a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in 







.jpg)






























 Editorial Board Registrations
Editorial Board Registrations Submit your Article
Submit your Article Refer a Friend
Refer a Friend Advertise With Us
Advertise With Us
.jpg)






.jpg)














.bmp)
.jpg)
.png)
.jpg)










.jpg)






.png)

.png)



.png)






