- Submissions

Full Text
Journal of Biotechnology & Bioresearch
Antibacterial Activity of Roots of Medicinal Plants from Flora of Pakistan
Zahid Nawaz1*, Anam Sajid2, Ejaz Ahmed1, Ahsan Sharif1, Muhammad Shahid1 and Nadeem Ullah3
1School of Chemistry, Faculty of Science, University of The Punjab, Lahore, Pakistan
2Department of Natural Sciences and Humanities, Faculty of NHS & IS, University of Engineering and Technology, Lahore, New Campus, KSK, Pakistan
2Department of Biotechnology, University of Central Punjab, Pakistan
*Corresponding author:Zahid Nawaz, School of Chemistry, Faculty of Science, University of The Punjab, Lahore, Pakistan
Submission: Febraury 20, 2023;Published: March 09, 2023

Volume4 Issue4March , 2023
Abstract
Medicinal plants have been used from centuries for human’s benefit. Due to immense importance of medicinal plants in human’s life this research project was designed to investigate the antibacterial activity of aqueous extracts from roots of eleven plants selected from flora of Pakistan. Experimental evidence showed that water extracts of all the roots were inactive except those of Z. officinalis (Ginger), A. sativum (Garlic), A. cepa (onion), Z. officinalis (sonth). The activity of water extracts of active roots was found to enhance with the increase in the concentration of the root materials. Out of 11 plant roots tested, four showed antibacterial activity against Escherichia coli and Staphylococcus aureus. The most active antibacterial species was Z. officinalis (Ginger), which completely inhibited the development of both E-coli and S-aureus. The water extracts of roots were found active against both the bacteria. Oil extracts of Ginger, Garlic and onion were also found to be active against E.coli and S. aureus. The residues left after oil extraction were found to be inactive.
Keywords: Antibacterial activity; Medicinal plant; Oil extracts; Traditional plants of Pakistan
Introduction
Medicinal plants are a common link between the traditional and modern medical science, as they are the main source of drugs used to cure large number of diseases [1]. Due to extensive scientific advancement, the synthetic drugs have replaced the conventional medicines throughout the world including the developing countries [2]. The use of medicinal plants originated from Egypt, China and Hindustan. Screening of compounds from plants is mostly to act as therapeutic agents [3,4]. These biological active molecules are fruitful in the field of antibiotics [5]. Medicinal plants are a source of providing antimicrobial agents or pathogenic agents [6-8]. These plants are used in various countries as a source of potent and drugs. Different parts of the plants i.e., roots, barks, leaves, fruits, seeds, and flowers have been investigated separately to determine the activity against different diseases. In some cases, roots or tubers and in others leaves, seeds or flowers have been found effective [9- 14]. Roots like ginger, garlic, onion, radish have been in use of man since long as effective remedies against different diseases [15-18]. Among diseases, infectious diseases are of great importance which is caused by the pathogenic micro-organism [8]. The medicinal plants or roots which have been used to cure infectious diseases must contain some factor which can kill these micro-organisms. This active factor may be oil or certain other chemical substances like Alkaloids, Steroids, Glycosides, Saponins, Resins, Tannins [19-21].
Antibacterial activity of various natural products has been studied by several researchers but most of them have investigated ethanolic fractions or some other non-aqueous fractions have been part of study. This research project focused on the antibacterial activity of water extracts from roots of various traditional plants of Pakistan including Zingiber officinalis (Ginger), Allium sativum (Garlic), Allium cepa (onion), Zingiber officinalis (sonth), Raphanus sativus (radish), Ipomea batatus (sweet potato), Brassia mapa (turnip), Curcuma longa (tumeric), Colocacssia antiguarum (oris) and Beta moritima (beet root). To the best of our knowledge antibacterial activity of aqueous extracts of all these extracts have not been published yet. All these compounds are being used in our daily life therefore, investigation regarding antibacterial activity of these extracts is of vital importance. Comparison of antibacterial activity of these samples is also part of this study.
Method and Materials
Collection of plant roots
All roots or tubers were collected from different shops in the Lahore city. These roots were washed thoroughly 3-4 times with running water at once with sterilized, distilled water then dried in air under shadow.
Micro-organisms
The tested species used in this work were Escherichia coli and Staphylococcus aureus. These species were collected from Post Graduate Research Centre, Mayo Hospital, Lahore, and from the Labex Clinic, Jail Road, Lahore.
Nutritive media
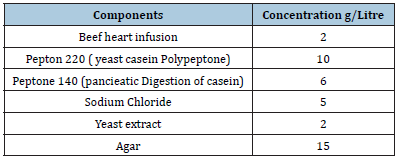
Blood agar base nutritive media is taken for the growth of E. coli and S. aureus. The main advantage of this medium is to maintain the low pH which is favourable for the multiplication of bacteria. The normal pH of this media is 6.8 ± 2 at 25 ºC. The composition of medium is given in Table 1.
Table 1:Composition of blood agar based medium used for growth of bacteria’s.
Preparation of plant material for antibacterial activity
Roots of all plants were treated in three different ways for investigating antibacterial activity.
Water extracts:The extraction was carried out by boiling the plant roots in an open pan with distilled water. 5gm of roots was boiled with 100ml of distilled water in 250ml beaker, at low flame till volume was reduced to half. The suspension was filtered off and extract was stored in a refrigerator.
Homogenized roots:The determination of antibacterial activity of roots after homogenization was conceived as there might be the possibility of the presence of volatile active components present in the roots and thus destroyed. Moreover, the sterilization at high temperature destroyed the active principal. 5gm of root sample was taken and ground to a powder form. This powder was directly used to determine antibacterial activity.
Extraction of active components by Soxhlet method: Extraction of active components was based upon the empirical knowledge. Soxhlet apparatus was used for extraction with ether and acetone. About 50gm root powder was taken and extraction in the first instance was carried out for 20 hours with 300ml of ether. The solvent was recovered by distillation and yield of oily residue calculated. Oil and residue both were stored in refrigerator.
Antibacterial Potential of roots using various extracts
Antibacterial activity of water extracts of roots: For determining antibacterial activity, 2gm of medium consisting of blood agar was dissolved in 50ml of aqueous extracts in a 250ml conical flask. The flask was cotton plugged, sterilized by autoclaving at 121 ºC and 15psi subsequently cooled to 60 ºC. 2ml of blood was transferred, and the contents of flask were shaken well. The medium thus constructed was poured into two sterilized petri dishes (one for Escherichia coli and other for Staphylococcus aureus) up to 2-3mm thickness. The contents were allowed to solidify. The Petri dishes were then ready for inoculation with the bacterial culture. To prepare control, 2gm of medium consisting of blood agar was dissolved in 50ml of distilled water and sterilized by autoclaving. The contents were cooled to 60 ºC and 2ml of blood was added. The medium thus prepared was poured into Petri dishes and allowed to solidify. Both test and control Petri dishes were inoculated with fresh bacterial cultures of E. coli and other for S. aureus and then incubated in a Gallen Kamp incubator at 37 ºC for 24 hours.
Antibacterial activity of different concentrations of root samples: 10gm, 15gm, 20gm, 25gm and 30gm of samples were boiled with 100ml of distil water respectively. The extracts of different concentrations of roots were thus prepared.
Antibacterial activity of homogenized roots: The root powder was added in blood agar base medium after sterilization and subsequent cooling to 60 ºC to avoid effects of high temperature on active components. 2gm of medium consisting of blood agar was dissolved in 15ml of distilled water and pasteurized by autoclaving at 120 ºC and 15psi. The contents were cooled to 60 ºC and 5gm of powder seeds and 2ml of blood were added. The medium thus prepared was shaken well and put into sterilized Petri dishes. Both sample and controlled Petri dishes were injected with bacterial cultures and incubated in Gallen kamp incubator at 37 ºC for 24 to 48 hours.
Antibacterial activity of oil extracts and root residue: Antibacterial activity of oil extracts and root residue was determined. Calculated quantities of extracts and residues were added after cooling blood agar base medium and then medium was contaminated with test bacteria to examine the bactericidal activity of the oil and the residue. Quantity of oil taken for the determination of antibacterial activity was proportional to the amount of the oil that might be present in 5gm of root material.
Results
Antibacterial activity of water extracts of roots
The results of experiments on antibacterial activity of water extract of roots against E. coli and S. aureus are recorded in the Table 2. Results show that Garlic, Ginger and onion are active against both organisms and Sonth were active only against E. coli. In the case of Ginger, there was no growth at all in the sample. However, in Garlic, Sonth and Onion growth was lesser than that in the control.
Table 2:Comparison of antibacterial activity of water extract of roots of various samples with control system.
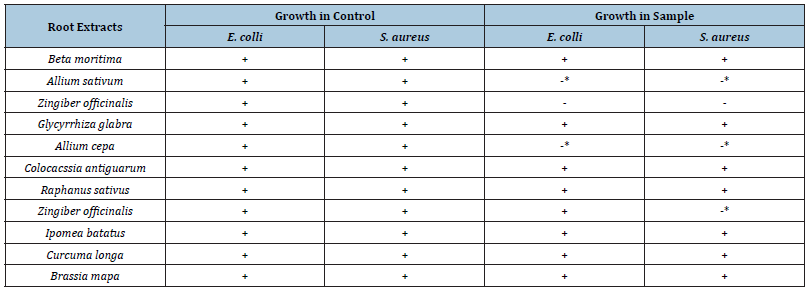
Note: -* this sign shows growth of microorganism but less than the control
Antibacterial activity with different concentrations of the samples extract
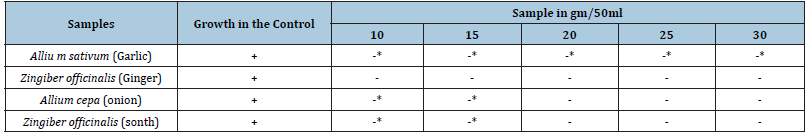
The results of experiments on antibacterial activity of water extracts of different concentrations of roots. Only one species has been taken for test i.e E. coli and four active species of plants i.e Garlic, Ginger, Onion and Sonth as shown in Table 3. This shows that the antibacterial activity increases with the increase in the weight of the root material. Sonth which was not antibacterial against E. coli with less concentration, but it becomes active by increasing the concentrations. There was complete blocking of bacterial growth in case of Ginger and Onion (Table 4,5)
Table 3:Antibacterial activity aqueous root extracts of various samples with different concentrations of samples.
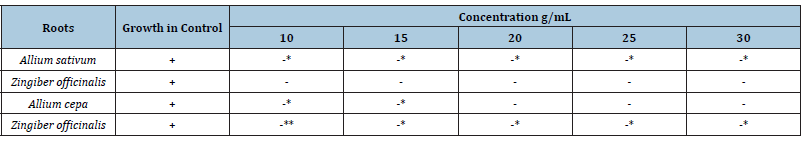
Note: -* This sign shows growth but less than control and -** shows growth much lesser than Control.
Table 4:Comparison of antibacterial activity of homogenized roots of various samples with control system.
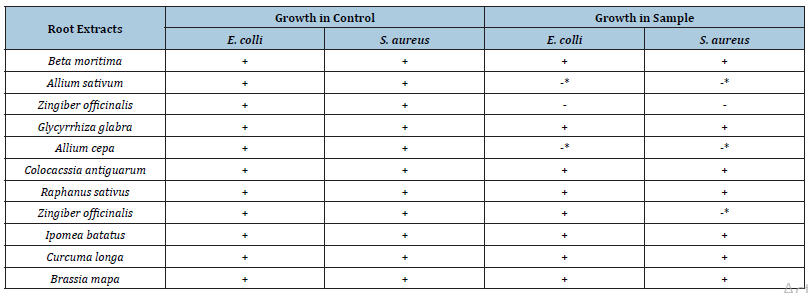
Note: -* this sign shows growth of microorganism but less than the control.
Table 5:Antibacterial activity of oil extract and root residue of various samples with control system.
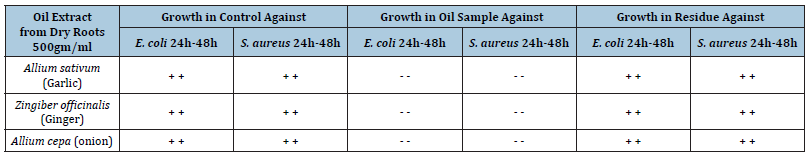
Note: – sign shows no appearance of bacterial colonies, + sign shows the growth of bacterial colonies.
Antibacterial activity of different concentrations of root samples against S. aureus
The results obtained from experiments about antibacterial activity of water extracts of different concentration of root materials has been examined as shown in Table 6. Results showed that the samples were more effective against S. aureus as compared to E. coli. There was no bacterial growth at all with higher weight sample of Ginger, Onion and Sonth. But in case of Garlic extracts bacterial growth has been observed.
Table 6:Antibacterial activity of aqueous extract of root samples at different concentrations with S. aureus.
Bactericidal activity of test samples against E. coli and S. aureus
Table 7:Antibacterial activity comparison between S. aureus and E.coli for various samples.
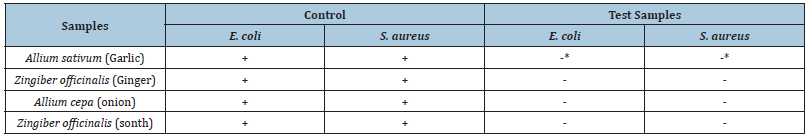
The results of bactericidal activity of test samples were determined as shown in Table 7. Disappearance of colonies occurred in all the samples except in the case of garlic.
Discussion
E. coli and S. aureus caused different diseases in human. For curing of diseases, medicinal plants are using. The root extractions of different plants are mainly used in Pakistan as medical treatments for their antibacterial actions. Water extracts of the roots were tested for antibacterial activity. Water extracts of Ginger, Garlic and Onion were found active against both organisms but Sonth was active only for S. aureus. Experimental procedure of water extracts, repeated with higher concentrations showed much better result. Garlic and Onion showed some growth of bacteria with less concentration, but completely inhibited the growth of bacteria with higher concentration. The water extract of Liquorice did not show activity while its powder was found active. This might be due to the reason that the active ingredient of the roots has been destroyed. During the process of preparation of the water extracts, Garlic, Ginger and Onion showed antibacterial activity. This has been further analysed to isolate the active principal. The result of antibacterial activity indicated that the oils extracted from all the three samples were active against both organisms. The residues were found inactive. This means that the active ingredients are extractable by polar solvents. Oils of Ginger, Garlic and Onion being active, it is recommended that instead of using original roots their oils rich in active ingredients should be applied for the treatment of infectious diseases.
References
- J Michel, Abd Rani NZ, Husain K (2020) A review on the potential use of medicinal plants from Asteraceae and Lamiaceae plant family in cardiovascular diseases. Frontiers in Pharmacology 11: 852.
- Banerjee M, Khursheed R, Yadav AK, Singh SK, Gulati M, et al. (2020) A systematic review on synthetic drugs and phytopharmaceuticals used to manage diabetes. Current Diabetes Reviews 16(4): 340-356.
- Bak JP, Kim JB, Park JH, Yang YJ, Kim IS, et al. (2011) Screening and compound isolation from natural plants for anti-allergic activity. Journal of the Korean Society for Applied Biological Chemistry 54(3): 367-375.
- Lankatillake C, Huynh T, Dias DA (2019) Understanding glycaemic control and current approaches for screening antidiabetic natural products from evidence-based medicinal plants. Plant Methods 15(1): 1-35.
- Savoia D (2012) Plant-derived antimicrobial compounds: Alternatives to antibiotics. Future microbiology 7(8): 979-990.
- Das K, Tiwari R, Shrivastava D (2010) Techniques for evaluation of medicinal plant products as antimicrobial agent: Current methods and future trends. Journal of medicinal plants research 4(2): 104-111.
- Tyagi R, Sharma G, Jasuja ND, Menghani E (2016) Indian medicinal plants as an effective antimicrobial agent. J Crit Rev 3(2): 69-71.
- Manandhar S, Luitel S, Dahal RK (2019) In vitro antimicrobial activity of some medicinal plants against human pathogenic bacteria. Journal of Tropical Medicine.
- Alam M, Gomes A (2003) Snake venom neutralization by Indian medicinal plants (Vitex negundo and Emblica officinalis) root extracts, Journal of Ethnopharmacology 86(1): 75-80.
- Helfer M, Koppensteiner H, Schneider M, Rebensburg S, Forcisi S, et al. (2014) The root extract of the medicinal plant Pelargonium sidoides is a potent HIV-1 attachment inhibitor. PLoS one 9(1): e87487.
- Ondua M, Njoya EM, Abdalla MA, McGaw LJ (2019) Anti-inflammatory and antioxidant properties of leaf extracts of eleven South African medicinal plants used traditionally to treat inflammation. Journal of ethnopharmacology 234: 27-35.
- Olela B, Mbaria J, Wachira T, Moriasi G (2020) Acute oral toxicity and anti-inflammatory and analgesic effects of aqueous and methanolic stem bark extracts of Piliostigma thonningii (Schumach.). Evidence-Based Complementary and Alternative Medicine 2020: 5651390.
- Indhumathi T, Mohandass S (2014) Efficacy of ethanolic extract of Solanum incanum fruit extract for its antimicrobial activity. International Journal of Current Microbiology and Applied Sciences 3(6): 939-949.
- Mohammed HA, Abdel Aziz MM, Hegazy MM (2019) Anti-oral pathogens of tecoma stans (L.) and cassia javanica (l.) flower volatile oils in comparison with chlorhexidine in accordance with their folk medicinal uses. Medicina (Kaunas) 55(6): 301.
- Shahrajabian MH, Sun W, Cheng Q (2019) Clinical aspects and health benefits of ginger (Zingiber officinale) in both traditional Chinese medicine and modern industry. Acta agriculturae scandinavica, section b-Soil & Plant Science 69(6): 546-556.
- Ashfaq F, Ali Q, Haider M, Hafeez M, Malik A (2021) Therapeutic activities of garlic constituent phytochemicals. Biological and Clinical Sciences Research Journal 2021: e007.
- Teshika JD, Zakariyyah AM, Zaynab T, Zengin G, Rengasamy KR, et al. (2019) Traditional and modern uses of onion bulb (Allium cepa L.): A systematic review. Critical reviews in Food Science And Nutrition 59(sup1): S39-S70.
- Anwar O, Iqbal M, Khan AM, Tariq S, Ambreen A (2020) Effect of Raphanus sativus (Radish) leaf extract and high doses of atorvastatin on body weight, liver weight and liver/body weight ratio. Annals of Punjab Medical College 14(4): 313-317.
- Uzor PF (2020) Alkaloids from plants with antimalarial activity: A review of recent studies. Evidence-Based Complementary and Alternative Medicine 2020: 8749083.
- Tlak IG, Dar SA (2021) Plant allelochemicals as sources of insecticides. Insects 12(3): 189.
- Sharma P, Tyagi A, Bhansali P, Pareek S, Singh V, et al. (2021 ) Extraction, bio-medicinal properties and way forward to anti-viral representatives. Food and Chemical Toxicology 150: 112075.
© 2023 Zahid Nawaz. This is an open access article distributed under the terms of the Creative Commons Attribution License , which permits unrestricted use, distribution, and build upon your work non-commercially.
 a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in
a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in 







.jpg)






























 Editorial Board Registrations
Editorial Board Registrations Submit your Article
Submit your Article Refer a Friend
Refer a Friend Advertise With Us
Advertise With Us
.jpg)






.jpg)














.bmp)
.jpg)
.png)
.jpg)










.jpg)






.png)

.png)



.png)






