- Submissions

Full Text
Journal of Biotechnology & Bioresearch
Isolation of Cellulolytic Micro-fungi from Rice Straw and Evaluation of Their Enzymatic Degradation Potential under Solid-State- Fermentation Conditions
Rinni Sahrawat and Amar P Garg*
School of Biological Engineering & Life Sciences, Shobhit Institute of Engineering & Technology, Deemed to-be-University, NH-58, Modipuram, India
*Corresponding author:Amar P Garg, School of Biological Engineering & Life Sciences, Shobhit Institute of Engineering & Technology, Deemed to-be-University, Modipuram, India
Submission: February 13, 2023;Published: February 22, 2023

Volume4 Issue4February , 2023
Abstract
We have isolated a large number of microfungi from naturally degrading rice straw (“Parali”) over a period of 3 months of incubation in and on the soil using moist chamber and dilution plate methods. On screening of all species for the production of CMCase activity on agar plates, 10 species of microfungi showed good activity. These included Aspergillus niger, A. flavus, A. oryzae, A. fumigatus, Penicillium chrysogenum, P. expansum, Chaetomium globosum, Fusarium oxysporum, F. rosesum and Trichoderma viride. All these 10 species were cultured for the production of cellulase activity under solid-state fermentation conditions. It was found that Aspergillus orzyae, A. flavus, A. fumigatus, Penicillium chrysogenum and Trichoderma viride showed high cellulases activity in terms of CMCase and FPase. Solid-state fermentation (SSF) medium included 5g rice straw in 500mL Erlenmeyer flask that was moistened with nutrient medium containing urea (1g/L), jaggery (5g/L) and yeast powder (2g/L) for the production of enzymes. Flasks were incubated at 28±2 °C for 14 days and the extracellular enzyme was recovered using citrate buffer and activity was measured in IU/mL using dinitros alicyclic (DNS) acid method. The highest cellulases activity of 37IU/mL was found with Penicillium chrysogenum followed by Aspergillus flavus 10.7IU/mL and A. oryzae 7.6IU/mL. It is suggested that micro-fungi may be used to degrade rice straw under field conditions to enhance its natural degradation to avoid the burning of “Parali” to fight with pollution.
Keywords: Decomposition; Rice straw; Cellulases; Solid state fermentation
Introduction
Lignocellulose being the major constituent of various agricultural waste residues is most abundant biomass on earth [1-3]. Lignocellulose usually comprise of three major polymeric components-cellulose(40-50%), hemicellulose (20-30%) and lignin (11-18%). Rice straw is relatively difficult to decompose in nature because of the presence of high amounts of silicon (9-14%) [4,5]. Huge amounts of lignocellulosic biomass are generated every year estimating to about more than 600 million tons by various agricultural practices and agro-based industries [6]. Hence, the farmers generally dispose its residue by burning in the field that produce smog in the air, losses the soil fertility and nutrients [7-9] and cause health hazards.
Several microbes (bacteria, fungi and actinomycetes) are known to degrade lignocellulose in nature and the mechanism of degradation of lignocellulose is well understood (Maria et al., 2022). Several microfungi are known to degrade lignocellulose [10-15] (Zhang et al. 2019). The microbial enzymes accelerate the rice straw decomposition in the field and improves the soil quality and fertility [16]. The lignocellulose has high potential for the production of ethanol using enzymatic process [17-20] where cellulase is converted into monomers (glucose) with the help of enzyme cellulases complex [21] which can then be fermented for the production of alcohol using a common yeasts
Solid state fermentation(SSF) and submerged fermentation are commonly used for the production cellulases enzyme [18,22,23]. SSF has great potential for the production of high activity of cellulases and require minimum facilities [24-26]. It has been reported that the solid-state fermentation(SSF) is an attractive alternative process to produce fungal microbial enzymes using lignocellulosic materials from the agricultural wastes due to its lower capital investment and lower operating costs [22,24,27,28]. The natural decomposition of rice straw is very slow and the immediate requirements of land for next crop, the farmers generally burn it in the field that create smog and pollution problems leading to several health hazards. Hence, the productions of high activity cellulases enzymes from different cellulolytic microbes is the logical scientific key for fast decompositions of rice straw [29]. Thus, the aim of the present study was to isolate high cellulolytic microfungi and to screen them for their bio-decomposition potential to explore the possibilities for their use to increase degradation of rice straw in the field.
Materials and Methods
Sampling
The samples of rice straw were collected in polythene from rice field of Rawali village near Muradnagar (U.P.) and were placed in twenty sterile Nylon net bags [10,11,30] (Wise and Schaefer.1994), each containing 20g of rice straw chopped into a 2-4cm pieces. Ten nylon bags with rice straw were buried 15cm below the soil surface and other 10 bags were kept on the soil surface for 2 months in the field of Shobhit Institute of Engineering & Technology, Meerut from March 2020-June 2020 as shown in Figure 1. Nylon bags with rice straw were taken out from pits at regular intervals of 15 days of incubation for 90 days (Figure 2) and the samples were collected and processed for the isolation of microfungi.
Figure 1:A: Nylon bags with rice straw outside the soil. B: Nylon bags inside the pits.

Figure 2:A: Nylon bags with rice straw outside the pits after 15 days. B: Nylon bags with rice straw inside the pits after 15 day of incubation. C: Rice straw after 60 days outside the pits. D: Rice straw after 60 days inside the pits.

Isolation of micro-fungi
Moist chamber method: Sterile moist chamber with 10cm diameter Petri dishes were prepared using moistened filter paper with two-glass slide placed in the center of the plate and autoclaved at 15p.s.i. for 15min. The samples collected from the nylon bags were placed separately at the center of glass slide and incubated at 28±2 °C to facilitate the growth of fungal species on decaying rice straw. These were examined using adhesive Cello tape technique described earlier by Garg et al. [31] where a small piece (1x1cm) of cello tape was gently pressed over the surface of the leaf that allowed the detachment of fungal spores with mycelium on the cello tape. It was then gently removed with the help of forceps and mounted on a plane microscope glass slide, stained with lactophenol+ cotton blue if necessary. The preparations were examined under binocular microscope (Leica EC 410X×40X) to identify the fungal species.
Dilution plating method:Disks (size 6mm diam.) were cut aseptically from the decaying rice straw samples using sterile Cork borer. Fifty disks were shaken in 100mL sterile distilled water in 250mL Erlenmeyer flask for 10 minutes and the suspension was treated as undiluted suspension. Dilution series with 1:10, 1:100 and 1:1000 was prepared and 1mL aliquot of each suspension was poured aseptically in each of the 10cm diam. sterile Petri plate to which 20mL of cool but molten Potato Dextrose Agar(PDA) medium containing 10μg/mL of streptomycin (to avoid bacterial growth) was poured aseptically with gentle shaking of the plate. The plates were incubated at 28 °C and 37±2 °C separately for 7 days. All plates were examined every day and the fungal colonies were picked up, sub-cultured on PDA and identified using standard reference books [32-37]. Pure cultures were maintained at 4 °C and on mineral oil.
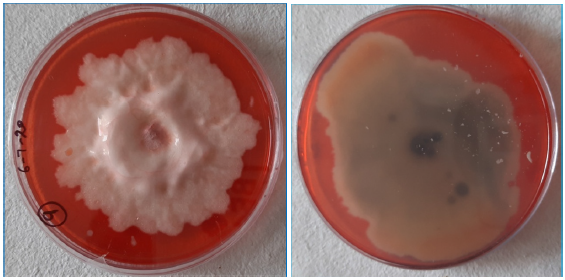
Enzymatic screening:10 of identified dominant fungal species were screened for visual cellulolytic activity using 2% (w/v) CMC (carboxy methyl cellulose) agar medium. A disk of test fungus was inoculated in the center of CMC agar plate aseptically and incubated at 28 °C and 37±2 °C separately in BOD incubator. The plates were examined for the production of clear halo zones on CMC plate (Figure 3) when it was treated with 1% Congo red dye for 15 minutes and rinsed with 1M NaCl.
Figure 3:Screening of fungi for the production celluloses enzyme complex on CMC Agar medium.
Production of cellulases enzyme complex under solid state fermentation (SSF) conditions:After preliminary screening 5 species of fungi were selected for the production of enzyme complex under SSF conditions. 5g of rice straw was added into 500mL Erlenmeyer flask to which 25mL of medium containing yeast powder (2g/L), jaggery (5g/L ) and urea (1g/L) were added to moisten the rice straw. These were autoclaved at 15psi for 15min and thereafter 5mL of spore suspension (O.D. 1 at 600nm) prepared from 5-day-old cultures of selected test fungal species, was inoculated aseptically. The flasks were incubated at 28±2 °C for 14 days (3 replicates for each species) and additional sterile water was added to keep the contents moistened if necessary. For extraction of the enzyme complex, 50mL citrate buffer (50mM, pH 4.8) was added to each flask and shaken at 100r.p.m. for 30min at room temperature. The contents were filtered and the filtrate was collected, centrifuged at 12,000xg at 4 °C for half an hour. The pellet was discarded and the filtrate was collected in Eppendorf tubes in 1 to 2mL aliquot, stored at -20 °C for further use for enzyme assay and treatment.
Enzyme assay
Endoglucanase activity was measured using 1% (w/v) carboxymethyl cellulose (CMC) at pH 4.8 with 0.05M sodium citrate buffer as a substrate suspension according to the procedure described by Ghose[38] and Garg et al. [39]. Dinitro salicylic acid (DNS) method [40] was used for determining reducing sugars. The control included with and without substrate and the reaction mixture included the substrate and enzyme at various dilutions and the best dilution showing no feedback inhibition was considered for calculations of IU/mL using the following formulae:

Degradation of rice straw in the field using individual species of micro-fungi
The mycelial and spore suspension of species showing high activities of CMCase and FPase were sprayed on rice straw kept on soil and were examined every 15 days for their degradation to find out the bio-decomposition potential of highly cellulolytic species.
Results and Discussion
Identification of fungal isolates
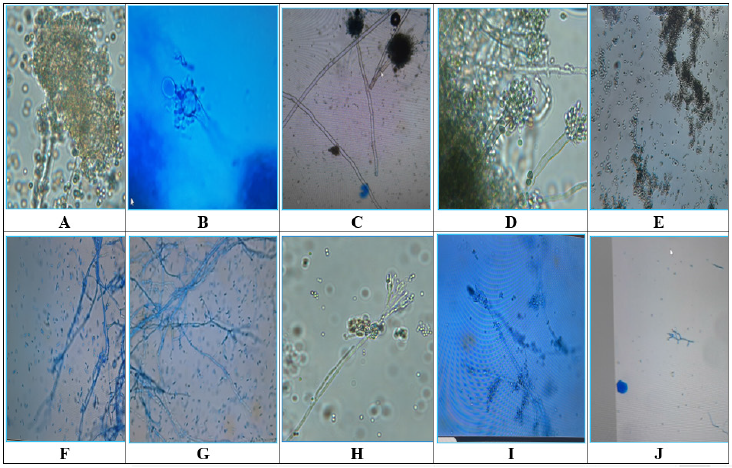
A total number of 36 species of fungi were isolated from decaying rice straw at various stages over a period of 90 days and were identified based on their morphological characters of spores, attachment of the spores, size of spores, color of mycelial colony, hyphal fragment, septation and other characters commonly used for the identification of fungi (Table 1) of which 10 were dominant as these were isolated in greater number and for longer duration. These included Aspergillus niger, A. flavus, A. oryzae, A. fumigatus, Penicillium chrysogenum, P. expansum, Chaetomium globosum, Fusarium oxysporum, F. rosesum and Trichoderma viride (Table 1, Figure 4).
Table 1:Isolation of dominant species of micro fungi from rice straw placed on and buried in the soil over a period of 60 days.
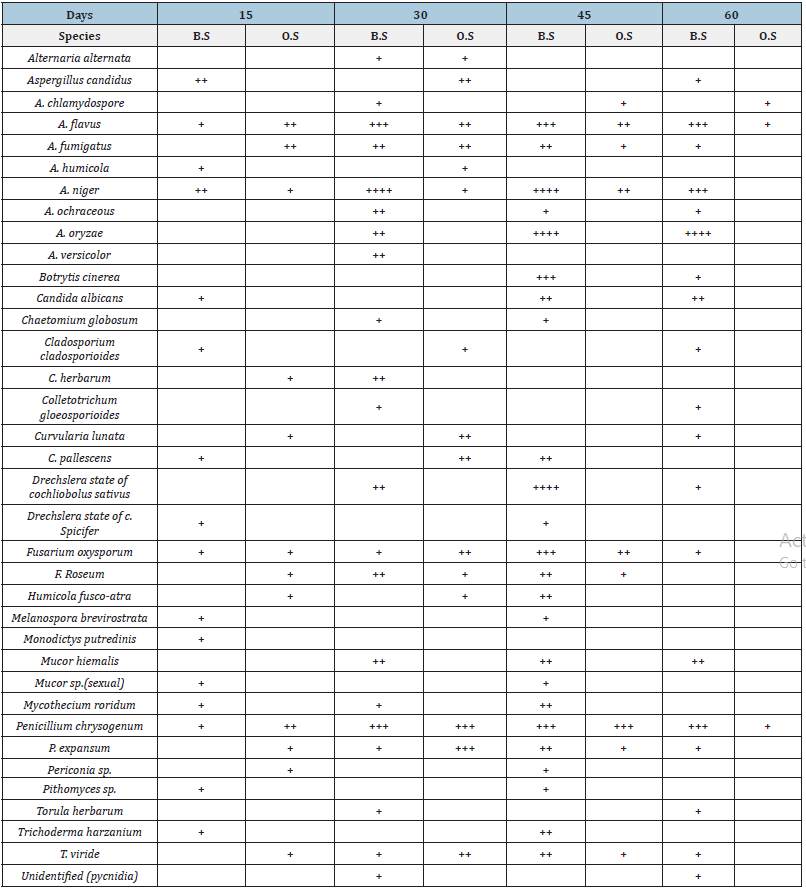
B.S. = buried in soil; O.S. = placed on soil
Based on preliminary screening on CMC Agar medium (Figure 2) for the production of endoglucanases enzyme, five species were selected for the production of cellulases complex enzyme under solid-state fermentation conditions. These included Aspergillus orzyae, A. flavus A. fumigatus, Penicillium chrysogenum and Trichoderma viride. The enzyme cellulases complex includes endoglucase, exolipase and β-glucosidases (Figure 4).
Figure 4:Microphotographs of dominant cellulolytic fungi (Original Maginification-10X×40X): A. Aspergillus flavus,Aspergillus fumigatus, B. Aspergillus niger, C. Aspergillus oryzae, D. Chaetomium globosum, E. Fusarium oxysporum, F. Fusarium roseum, G. Penicillium chrysogenum, H. Penicillium expansum, I. Trichoderma viride J. Trichoderma viride
All these 10 dominant species were screened for the production of high activity of cellulases using 2%CMC agar plates and 5 species were selected based on clear halo zones produced by the test species (Figure 3).
These included Aspergillus flavus, A. fumigatus, A. oryzae, Penicillium chrysogenum and Trichoderma viride. These selected species were cultured on 5% rice straw under solid-state fermentation conditions for 14 days at pH 7.0 and temperature 30±1 °C and the cellulases activity in terms of CMCase and FPase were determined (Table 2). On comparison, it was found that Penicillium chrysogenum showed highest CMCase activity of 37.0 IU/mL followed by Aspergillus flavus, A. oryzae, A. fumigatus and Trichoderma viride (Table 2). Spores and mycelial suspension of these species were sprayed on rice straw placed on the soil and the visual degradation was observed after every 15 days. It was found that Penicillium chrysogenum degraded the rice straw completely within 45 days while Aspergillus flavus took 60 days to degrade the rice straw in the field. A. oryzae, A. fumigatus and Trichoderma viride could finally degrade the rice straw within 90 days, double the period taken by Penicillium chrysogenum. It suggests that P. chrysogenum has highest bio-degradation potential for rice straw and can be used for natural degradation of “parali” in the fields to combat the pollution by avoiding its burning in the fields.
Table 2:CM Case and FPase activity of selected micro fungi isolated from rice straw and the number of days taken by fungus to degrade rice straw in the field.
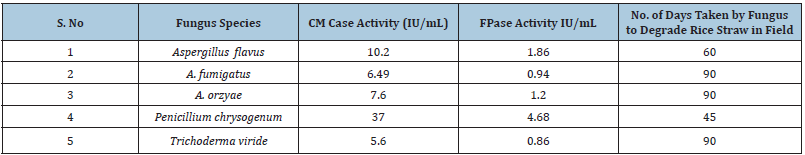
Cellulases is a complex group of enzymes that mainly include β-1, 4-endoglucanases, cellobiohydrolases, and β-glucosidases and they break down different bonds at different positions in complex glucose polymer of cellulose. These enzymes are further divided into different sub-groups based on their molecular weights and structure. Different enzymes are produced in different amounts with varying activities by various microbes on different cellulosic materials that complicate the production of cellulolytic enzyme complex and bioconversion of cellulosic material in nature as well as in the laboratory. The scientists are working on production of high activity cellulases using submerged and solid-statefermentations processes. Submerged fermentation require lesser quantities of substrate but produce lesser active enzyme complex while solid-state fermentation (SSF) allows the use of 2 to 5% of the substrate and gives higher yield of 50-100 times highly active enzyme complex. Further, SSF requires lesser complicated equipment and maintenance [22,24]. Solid-state-fermentation technology has enabled the scientists to use higher concentrations of substrate for the production of high activities of enzymes [24]. SSF is generally recommended if the cost of the raw material is less or negligible. Cellulose is abundantly present in nature and is a renewable resource with free availability at much lesser cost as agricultural waste amounting to annual production of 600 million tons of biomass in India with 140 million tons of rice straw alone [41]. In view of the merits of SSF, we used it for the production of high activity cellulases using Aspergillus flavus, A. oryzae, A. fumigatus, Penicillium chrysogenum and Trichoderma viride. Since, rice straw is a waste agricultural product and the farmers burn it in the field to vacate the land for sowing the next crop, hence, the abundant availability of the rice straw as raw material made the basis of the use of SSF for the production of cellulases complex in the present paper. The results revealed that Penicillium chrysogenum produced highest activity of CMCase of 37.0IU/mL with 4.68FPase and degraded the rice straw in the field just within 45 days and Aspergillus flavus with CMCase activity of 10.2 and FPase 1.86 activity took 60 days to degrade the rice straw while all other three species showed much lower activities of CMCase and FPase and took double the time of 90 days for the degradation of rice straw in the field in comparison to P. chrysogenum (Table 2). Fast microbial degradation of rice straw using microbial cultures spray in the field as an alternate process to attract the farmers for natural degradation of rice straw will discourage them from burning it in the fields. The process will ensure the increase of soil fertility also, however, still more research is required so as to study the impact of additional spray of P. chrysogenum on population of other natural species of microbes in different soils. Fungi have the ability to decompose cellulose, hemicellulases and lignin in plants by secreting various sets of hydrolytic and oxidative enzymes. Synergistic action of cellulose degrading enzymes endoglucanases, exoglycanases, cellobiohydrolases and glucosidases effectively biodegrade cellulase to glucose [12]. Species from the genus Penicillium have been described as potential producers for commercial cellulases (Saini et al. 2015). Berlemont et al. [42] while studying the cellulolytic potential of microbial communities under environmental changes found that microbial taxonomic composition was predictive of cellulolytic potential. Thota et al. [43] isolated various species of micro and macrofungi from groundnut shell that produced high cellulolytic activities under SSF conditions where they used mixed cultures of Aspergillus oryzae, Trichoderma harzianum and Pycnoporuous sanguineus. Akpomie et al. [44] have also demonstrated that Aspergillus flavus is highly cellulolytic that was isolated from compost pit. Nguyen et al. [45] used polypore’s macrofungi for production of high activity cellulase using SSF and found that Microporus sp. KA038 has great potential for commercial exploitation. It, is therefore, concluded that micro and macrofungi can be used for the production of high activity cellulases and these can also be used to accelerate the process of degradation of agricultural waste in the soil to improve the soil fertility, however, more researches are required to find out the interaction of added species of fungi with natural populations of microbes in the soil [46-49].
Conclusion
Our studies revealed that Penicillium chrysogenum produced highest activity of CMCase (37.0IU/mL) under solid-statefermentations( SSF) conditions followed by Aspergillus flavus (10.2IU/mL), A. oryzae (7.6IU/mL), A. fumigatus (6.49IU/mL) and Trichoderma viride (5.6IU/mL) on rice straw when 5% substrate was used. The spores and mycelial spray of Penicillium chrysogenum degraded the rice straw in just 45 days while A. flavus took 60 days to degrade it. On the other hand, A. fumigatus, A. oryzae and Trichoderma viride degraded the rice straw in 90 days. It is concluded that P. chrysogenum may be used to degrade the rice straw in the field to discourage the farmers to burn in.
Acknowledgement
The authors are grateful to Shobhit Institute of Engineering and Technology for financial support for completion of the present work.
References
- Shanmugapriya M, Lakshmiprabha M (2013) Bio bleaching and delignification of hard wood kraft pulp by white rot fungi. Int J Pharm Chem Sci 2(2): 932-937.
- Masran R, Zanirun Z, Bahrin EK, Ibrahim MF, Lai Yee P, et al. (2016) Harnessing the potential of ligninolytic enzymes for lignocellulosic biomass pretreatment. Appl Microbiol Biotechnol 100(1): 5231-5246.
- Shirkavand E, Baroutian S, Gapes DJ, Young BR (2016) Combination of fungal and physicochemical processes for lignocellulosic biomass pretreatment -A Review. Renew Sustain Energy Rev 54: 217-234.
- Chang C, Zhang L (2011) Cellulose based hydrogels present status and application prospects. Carbohydrate Polymer 84(1): 40-53.
- Rashad R, Hussien R ( 2013) Studying the use of cellulose, silica and lignin extracted from rice straw as sandy soil conditioners. International Journal of Agronomy and Agricultural Research 3(12): 21-35.
- Tu WC, Hallett JP (2019) Recent advances in the Pretreatment of Lignocellulosic Biomass. Current Opinion in Green and Sustainable Chemistry 20: 11-17.
- Satlewal A, Agrawal R, Bhagia S, Das P, Ragauskas AJ (2018) Rice straw as a feedstock for biofuels: Availability, recalcitrance, and chemical properties. Biofuels Bioprod Bioref 12(1): 83-107.
- Dhakate SR, Pathak AK, Jain P, Singh M, Singh BP, et al. (2019) Rice straw biomass to high energy yield biocoal by torrefaction. Current Science 116(5): 831-838.
- Pandey P (2020) RRI’s commitment to care and vulnerability of agrarian systems: The problem of rice straw burning in India. Sci Technol Soc 25(2): 240-255.
- Garg AP, Sharma PD (1984) Ecology of phylloplane and litter fungi of triticale. Nordic J Bot 4(5): 707-715.
- Garg AP, Sharma PD (1985) Ecology of phylloplane and leaf-litter fungi of Cyamopsis tetragonoloba c (L.) Taub. Rev Ecol Biol Sol 22: 35-55.
- Lynd LR, Weimer PJ, van Zyl WH, Proterius IS (2002) Microbial cellulose utilization: fundamentals and biotechnology. Microbiology and molecular biology review 66(3): 506-577.
- Bischof RH, Ramoni J, Seiboth B ( 2016) Celluloses and beyond: the first 70 years of the enzyme producer Trichoderma reesei. Microb Cell Fact 15: 106.
- Wang X, He P, Xu X, Qiu S, Shicheng Z (2022) Characteristics of rice straw decomposition and bacterial community succession for 2 consecutive years in a paddy field in Southeastern China. Sci Rep, Volume 12(1): 20893.
- Durairaj S, Gunasekaran S, Poovanalingam TV, Durairaj R, Ahmed A, et al. (2022) Enhanced cellulase enzyme production by Aspergillus niger using cellulase/iron oxide magnetic nano-composites. Journal of King Saud University-Science 34(1): 101695.
- Dhinu Y, Leelawati (2019) Bioconversion of rice straw into ethanol: Fungi and yeasts are the backbone microbiota of the process. International Journal of Current Microbiology and Applied Sciences 8(9): 913-920.
- Pandey S, Garg AP (1995) Assessment of decomposition potential of some dominant fungi isolated from barley. J Indian bot Soc 75: 345-346.
- Singh J, Garg AP (1995) Production of celluloses by Gliocladium virens Miller et al. on Eichhornia under solid-state- fermentation conditions. J Indian bot Soc 75: 305-309.
- Kumari D, Singh R (2018) Pretreatment of lignocellulosic wastes for biofuel production. A critical review. Renew Sustain Energy Rev 90: 877-891.
- Bolaji OJ (2019) Fungal treatment of selected matured forages to improve their value for ruminant feeding. School of Natural and Environmental Sciences Newcastle University, England, UK.
- Milala MA, Shugaba A, Gidado A, Ene AC, Wafar JA ( 2005) Studies on the use of agricultural wastes for cellulase enzyme production by Aspergillus niger. Journal of Agriculture and Biological Science 1(4): 325-328.
- Chahal DS (1985) Solid state fermentation with Trichoderma ressei for cellulase production. Appl Environ Microbial 49(1): 205-210.
- Dasari PR, Ramteke PW, Kesri S, Kongala PR (2019) Comparative study of cellulase production using submerged and solid-state fermentation. Approaches to Enhance Industrial Production of Fungal Celluloses, pp. 37-52.
- Singh J, Garg AP (1996) Bioconversion of Eichhornia leaf-litter using Gliocladium virens. Acta Bot Indica 24 : 67-71.
- Delabona DS, Pirota PRDB, Codima CA, Tremacoldi CR, Rodrigues A, et al. (2012) Using amazon forest fungi and agricultural residues as a strategy to produce cellulolytic enzymes. Biomass Bioenergy 37: 243-250.
- Coradi GV, Visitação VLD, De Lima EA, Saito LYT, Palmieri DA, et al. (2013) Comparing submerged and solid-state fermentation of agro-industrial residues for the production and characterization of lipase by Trichoderma harzianum. App Microbial 63: 533-540.
- Haltrich D, Nidetzky B, Kulbe KD, Walter S, Silvia Ž (1996) Production of fungal xylanases. Bioresource Technology 58(2): 137-161.
- Jech L (2000) Solid-state fermentation of agricultural wastes for endoglucanase production. Industrial Crops and Products 11(1): 1-5.
- Thota SP, Badiya PK, Guragain YN, Vadlani PV, Pandey M, et al. (2018) Innovative consortia of micro and macro fungal systems: Cellulolytic enzyme production from groundnut shell biomass and supportive structural analysis. Journal of Sustainable Bioenergy Systems 8(3): 47-66.
- House GT, Stinner RF (1987) Decomposition of plant residues into tillage agroecosystems. Influence of litter on mesh size and soil arthropods. Pedobiologia 130: 351-360.
- Garg AP, Sainger DK, Sharma PD (1978) Phylloplane micro fungi of barley, triticale and eggplant. Acta Bot Indica 6(suppl 1): 32-40.
- Subramanian CV (1971) Hyphomyc`e New Delhi: Indian Council of Agricultural Research, India.
- Gilman JC (1957) A manual of soil fungi. Oxford & IBH Publishing Co. New Delhi, India, p. 450.
- Ellis MB (1971) Dematiaceous hyphomycetes. Commonwealth Mycological Institute Kew, England, UK, p. 608.
- Ellis MB (1976) More dematiaceous hyphomycetes. Commonwealth Mycological Institute, Kew, Surrey, England, UK, pp. 507.
- Barnett HL, BB Hunter (1972) Illustrated genera of imperfect fungi. (4th Edn), Burgess Publishing Co, Minnesoha, USA, p. 241.
- Garett SD (1963) Soil fungi and Soil fertilty. Oxford Peragamon Prees.
- Ghose TK (1987) Measurement of cellulase activities. Pure and Applied Chemistry 59(2): 257-268.
- Singh S, Garg A, Singh J (1996) Bioconversion of pea crop residues using Gliocladium virens. J Indian bot Soc 75: 245-250.
- Miller L (1959) Use of dinitro salicylic acid reagent for determination of reducing sugar. Analytical Chemistry 31(3): 426-428.
- Dhakate SR, Pathak AK, Jain P, Singh M, Singh BP, et al. (2019) Rice straw biomass to high energy yield bio coal by torrefaction- Indian Perspective. Curr Sci, pp. 1-8.
- Berlemont R, Allison SD, Weihe C, Lu Y, Brodie EL, et al. (2014) Cellulolytic potential under environmental changes in microbial communities from grassland litter. Frontiers in microbiology 5: 639.
- Thota Sai, Praneeth B, Pradeep K, Guragain Y, Vadlani P, et al. (2018) Innovative consortia of micro and macro fungal systems: cellulolytic enzyme production from groundnut shell biomass and supportive structural analysis. Journal of Sustainable Bioenergy Systems 8(3): 47-66.
- Akpomie OO, Okonkwo KE, Gbemre AC, Akpomie KG, Ghosh S, et al. (2021) Thermotolerance and cellulolytic activity of fungi isolated from soils/waste materials in the industrial region of Nigeria. Current Microbiology 78(7): 2660-2671.
- Nguyen K, Penkhrue W, Lumyong S (2019) Production of high cellulase yields from polypore fungi in solid-state fermentation using green tea waste. BioRxiv.
- Gmoser R, Sintca C, Taherzadeh MJ, Lennartsson PR (2019) Combining submerged and solid state fermentation to convert waste bread into protein and pigment using the edible filamentous fungus N. intermedia. Waste management 97: 63-70.
- Gurovic MSV, Viceconte FR, Bidegain MA, Dietrich J (2023) Regulation of lignocellulose degradation in microorganisms. Journal of Applied Microbiology 134(1): lxac002.
- Štursová M, Žifčáková L, Leigh MB, Burgess R, Baldrian P (2012) Cellulose utilization in forest litter and soil: Identification of bacterial and fungal decomposers. FEMS Microbiology Ecology 80(3): 735-746.
- Patel RA, Patel KC, Malav JK (2017) Status of silicon in rice (Oryza sativa ) and its correlation with other nutrients under Typic ustochrepts soil. Int J Curr Microbiol App Sci 6(12): 2598-2611.
© 2023 Amar P Garg. This is an open access article distributed under the terms of the Creative Commons Attribution License , which permits unrestricted use, distribution, and build upon your work non-commercially.
 a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in
a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in 







.jpg)






























 Editorial Board Registrations
Editorial Board Registrations Submit your Article
Submit your Article Refer a Friend
Refer a Friend Advertise With Us
Advertise With Us
.jpg)






.jpg)














.bmp)
.jpg)
.png)
.jpg)










.jpg)






.png)

.png)



.png)






