- Submissions

Full Text
Journal of Biotechnology & Bioresearch
Cistus ladanifer L. Tissue Culture from Leaf and Stem Explants
David Franco Frazão1,2, Celina Barroca2,3, Amélia M Silva1,4, Fernanda Delgado2,3,5 and José Carlos Gonçalves2,3,5*
1Center for Research and Technology of Agro-Environmental and Biological Sciences (CITABUTAD), University of Trás-os-Montes e Alto Douro, Quinta de Prados, 5001-801 Vila Real, Portugal
2Plant Biotechnology Center of Beira Interior (CBPBI), Quinta da Senhora de Mércules, Apartado 119, 6001-909 Castelo Branco, Portugal
3Polytechnic Institute of Castelo Branco-Agronomics School (ESA-IPCB), Quinta da Senhora de Mércules, Apartado 119, 6001-909 Castelo Branco, Portugal
4Department of Biology and Environment; University of Trás-os-Montes e Alto Douro (UTAD), Quinta de Prados, P-5001-801 Vila Real, Portugal
5Research Centre for Natural Resources, Environment and Society (CERNAS-IPCB), Instituto Politécnico de Castelo Branco, Portugal
*Corresponding author:José Carlos Gonçalves, Plant Biotechnology Center of Beira Interior (CBPBI), Polytechnic Institute of Castelo Branco-Agronomics School (ESA-IPCB), Research Centre for Natural Resources, Environment and Society (CERNAS-IPCB), Quinta da Senhora de Mércules, Apartado 119, 6001-909 Castelo Branco, Portugal
Submission: November 11, 2022;Published: November 28, 2022

Volume4 Issue3November , 2022
Abstract
Cistus ladanifer L. exudes a phenolic and terpenoid resin with interesting bioactive and aromatic properties. Despite its high abundance in the wild, this plant can be cultivated to advantage on oligotrophic and trace-elements contaminated soils. Plant tissue culture may be used to produce specific metabolites or for clonal propagation of specific genotypes for plantation. From a biotechnological perspective this is the second study that has attempted in vitro propagation of C. ladanifer from adult plant material. Its goal was to evaluate the potential of leaf and internodal stem explants from C. ladanifer for in vitro tissue culture. Three plant growth regulators were tested: 2,4-Dichlorophenoxyacetic acid (2,4-D), 6-Benzylaminopurine (BAP), and 1-Naphthaleneacetic acid (NAA). From both explants, shoots were regenerated under the influence of BAP (38%) and two types of compact calli were induced: dark green calli were induced under the influence of BAP (above 70%) and light green calli were induced under the influence of 2,4-D with or without BAP (100%). Light green calli grew between 558 and 708% during subsequent subcultures and showed rhizogenic capacity when the amounts of BAP were lower than of 2.4-D, but they showed low potential for shoot organogenesis. Dark green calli were associated with shoot organogenesis. The suitability of the two calli lines to produce metabolites and their transposition to liquid cultures is worth further study in comparison to organ in vitro cultures.
Keywords: Callogenesis; Callus Histology; Cistaceae; In Vitro propagation; Organogenesis; Rockrose
Introduction
Cistus ladanifer L. is an endemic and abundant plant resource in the Iberian Peninsula [1]. Resin (labdanum) and extracts from this plant have been evaluated with regards to their bioactivities and have shown to have potential for medicinal, cosmetic, preservative and plant protection uses, besides their current use in perfumery and fragrances [2]. Raimundo et al. [2] suggested this plant could be used to recuperate oligotrophic soils and be cultivated in tracemetal contaminated soils which highlights the need to select specific plant lines.
In vitro plant tissue culture is a biotechnological alternative for plant propagation and/ or metabolite production [3]. Propagation of C. ladanifer is important once specific genotypes are selected for cultivation purposes. This plant propagates in nature by seeds resulting from cross pollination [4]. Using temperature (e.g., 90 °C) during a short time (e.g., 10min) to break dormancy it is possible to obtain near 100% of germination [5]. However, due to the cross-pollination process, seeds are not ideal to propagate specific genotypes. Vegetative propagation was attempted before this study, using terminal cuts with or without auxin (indol-3-butiric acid, IBA) supplementation during Spring. After two months, high mortality (above 50%) was registered although roots formation was high in viable cuts (above 75%) (data not published). Micropropagation has the advantage of producing large numbers of homogeneous plants at any time, generation of disease-free and aseptic plants and enhancement of multiplication rates. Iriondo et al. [6] developed a micropropagation method for several Cistus species using nodal segment explants from in vitro germinated seedlings. Boukili et al. [7] were able to induce shoot formation from nodal segments, from wild plants, highlighting the need for a pre-treatment with an antioxidant solution. Iriondo et al. [6] and Boukili et al. [7] report the advantage of using BAP over Kin or 2iP for shoot multiplication, the use of IBA over NAA to increase rooting, and achieving survival rates between 62 and 70% respectively, during the acclimatization phase.
Production of labdanum resin by in vitro systems may not be competitive with wild collection because of the resource abundance. However, the need to produce specific compounds, since some may show interesting individual bioactive properties, can justify in vitro culture systems in which the secondary metabolism could be easier to manipulate. Plant tissue culture advantages over field culture or natural exploitation for metabolite production are the following: independence from geographical, seasonal and environmental variations; uninterrupted production in uniform yield and quality; no need for pesticide and herbicide application; and shorter growing cycles [3]. However, a significant disadvantage are the higher costs involved [3]. Callus culture may be used as a source material for protoplast isolation (ideal to produce hybrids, genetic and cytoplasmatic manipulation); as a source of cells for genetic manipulation; as inoculum for the initiation of suspension cultures (ideal to produce metabolites); to provide information on the regeneration potentiality of a plant [8]; and for propagation purposes. In fact, according to Efferth [9] callus cultures and cell suspension cultures have a high potential use in pharmaceuticals production and for plant breeding. Callus culture and secondary metabolite production by plant tissue culture using C. ladanifer is not yet reported in literature. Nevertheless, Madesis et al. [10] regenerated shoots from Cistus creticus leaf explants through organogenesis from callus using a combination of TDZ and NAA, and Skorić et al. [11] were able to obtain extracts rich in labdanetype diterpenoids with cytotoxic activity against human cancer cell lines from C. creticus shoot cultures but the same did not happened with C. creticus root cultures. The aim of this work was to evaluate the potential of leaf and internodal stem explants from C. ladanifer to obtain in vitro tissue cultures for posterior clonal propagation and secondary metabolite production.
Material and Methods
Leaf and stem explants establishment and callogenesis
Non-flowering, semi-woody/herbaceous, dolichoblast shoots of C. ladanifer were collected from plants in a natural shrubland in Penha Garcia, Castelo Branco, Portugal (GPS 40o01’43.5’’N 6°59’35.4’’W) in April. The plant material was transferred to the laboratory immediately after excision and rinsed with flowing tap water for 20min. Subsequently the material, leaves and leafless shoots, was surface sterilized under a laminar air flow cabinet, by applying 70% ethanol for 1min, fungicide (Mancozeb® solution, 0.5g L?-1) for 10min, and then with a mercury 0.08% (w/v) HgCl2 for 4min. Finally, they were rinsed with sterile distilled water 5 times. The sterilized internodal segments and leaf segments (both basal and apical with 2cm length) were cut and used as explants on Murashige and Skoog [12] basal medium with 20g L-1 sucrose solidified with 8g L-1 agar. Additionally, different concentrations and combinations of 6-benzylaminopurine (BAP), 1-naphthaleneacetic acid (NAA), and 2,4-dichlorophenoxyacetic acid (2,4-D) were added to the medium for establishment, callus culture and organogenesis. pH was adjusted to 5.7±0.2. All cultures were incubated at 25±2 °C, during 45 days with a 16h photoperiod (“cool white” fluorescent lamps, 45±5μmol m-2 s-1 PPFD). Twenty-four leaf and stem explants were used per treatment (leaf explants were divided into twelve basal and apical explants per treatment). For 45 days, events such as contaminations, death/survival, and induction responses (callus and organ formation) were registered. Contamination percentage was calculated in relation to the total number of explants used, viability percentage was calculated in relation to the number of explants not contaminated, and induction responses percentage were calculated in relation to the number of viable explants.
Callus index (CI)
CI was calculated based on Wakhlu [13] method to evaluate the treatments at the callogenesis stage. Calli were visually rated (G) at a scale from 1 to 3 (1: callus initiation; 2: callus covering partially the explant; 3: callus covering completely the explant). CI was calculated, per treatment, based on the average calli rate (G ̅), the number of explants with callus (n) and the total number of explants, according to the formula: CI=100nG ̅(1/N).
Callus subculture and mass propagation
The treatment with 0.5mg L-1 BAP and 0.5mg L-1 2,4-D was selected to induce callogenesis. Ten calli lines were evaluated during a further five 30-day subcultures, in medium supplemented with 0.5mg L-1 2,4-D and two different concentrations of BAP (0.25 and 0.5mg L-1), to find the best combination for callus proliferation. All other culture conditions were the same as for establishment. Four non-necrotic/oxidized and non-differentiated pieces of calli were placed in a flask on 50mL of medium, corresponding to one callus line. Remaining portions were used for dry weight (DW) and histological analysis. Fresh (FW) and dry weight of the initial and final calli mass per calli line was monitored and reported as percentage of growth. After each subculture a portion of each callus line was initially weighted, dried in a ventilated chamber at 105 °C for 1 day and finally weighted to assess the dry weight content. Calli growth was morphologically evaluated regarding texture, color, necrosis/oxidation, and differentiation. Callus growth from control was excluded due to lack of growth right after the first subculture.
Histologic evaluation
From the fourth subculture, callus portions without any differentiation from the 3 treatments and callus portions with differentiated structures (Figure 1G-J) were immediately immersed in FAA solution (formaldehyde 37% v/v: glacial acetic acid: ethanol: water 2:1:10:7 v:v:v:v), for fixation, during 24h. Then, samples were rinsed twice with 50% ethanol during 2h and, after that, dehydrated with subsequent higher concentrations of ethanol (60, 70, 80, 90, 99%) for 1h each and 24h for the last. Infiltration was done with hydroxyethyl-methacrylate under vacuum, firstly in a 50% solution during 2h and finally in pure resin during 3h. Inclusion was done using the same resin and dimethyl sulfoxide as a polymerization agent during 2h at ambient temperature. Cuts were done at 7μm thickness using a rotative microtome (American Optical Company, model 820). Staining was done with 0.05% toluidine blue for 1 minute. Slides were visualized in a Motic BA 310 Digital microscope and photographs were taken using Motic Images Plus 2.0 software.
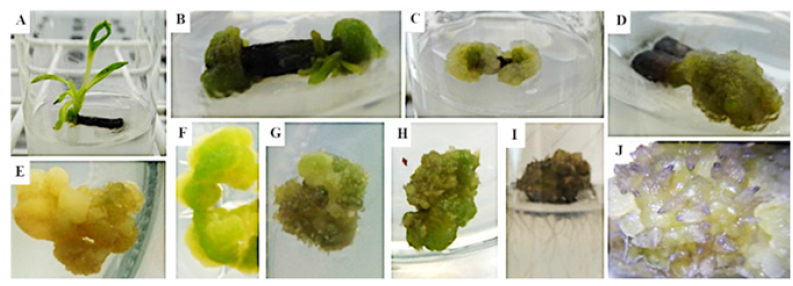
Figure 1: Morphological evaluation of the leaf and shoot establishment and callus culture.
A: shoot differentiation from a stem explant in 0.5mg L-1 BAP supplemented medium;
B: Compact dark green callus plus shoot differentiation from a stem explant in 0.5mg L-1 NAA + 0.5mg L-1 BAP
supplemented medium;
C: Compact light green callus from a stem explant in 0.5mg L-1 2,4-D + 0.5mg L-1 BAP supplemented medium;
D: Compact green callus from a leaf basal explant in 0.5mg L-1 NAA + 0.5mg L-1 BAP supplemented medium;
E: Compact yellow/white callus from 0.5mg L-1 2,4-D + 0.25mg L-1 BAP supplemented medium subculture;
F: Compact green callus from same medium subculture;
G and H: Compact green callus with root-like differentiation from 0.5mg L-1 2,4-D supplemented medium subculture;
I: Callus with true roots, result from a side experiment were calli from 0.5mg L-1 2,4-D + 0.25mg L-1 BAP
supplemented medium were subcultured without removing differentiation structures;
J: Root-like differentiation detail using a magnifying glass from callus on 0.5mg L-1 2,4-D + 0.25mg L-1 BAP
supplemented medium.
Shoot organogenesis
Five calli lines (n=20) obtained in medium with 0.5mg L-1 BAP and 0.5mg L-1 2,4-D were evaluated during two consecutive 30-day subcultures, in medium supplemented with 0.5mg L-1 NAA and 0.5mg L-1 or 1mg L-1 BAP to find the best combination to induce shoot organogenesis. All other culture conditions were the same as for establishment. Four non-necrotic/oxidized and nondifferentiated pieces of calli were placed, in a flask, with 50mL of the same medium of origin, corresponding to one callus line. During the two subcultures, induction responses (differentiation into shoots or roots) were registered.
Statistical analysis
Treatments were set up in a completely randomized design and the number of explants per treatment is given in the results. Statistical analysis, namely Pearson’s correlation test, ANOVA and post-hoc Tuckey test, and the non-parametric Kruskal-Wallis test were performed using IBM SPSS Statistics V.25 software, with a significance level (α) of 0.05.
Result and Discussion
Establishment of leaf and internodal stem explants
Percentage of contaminations, survival and induction responses of leaf and stem explants during establishment are reported as percentage of the events in Table 1. The explant preparation method showed a disinfection efficiency of about 70%. In addition, the method does not seem to influence the explants’ survival because of the high survival rates of leaf basal segments (94%) and internodal segments (77.4%). However, leaf apical segments showed a very low survival rate (13%).
Table 1: Percentage of contamination, survival, and effects of different plant growth regulators on induction of morphogenic responses observed for the leaf and stem explants establishment of C. ladanifer. Twenty-four explants (internodal or leaf) were used per treatment. Leaf explants in each treatment were composed by twelve basal and apical explants.
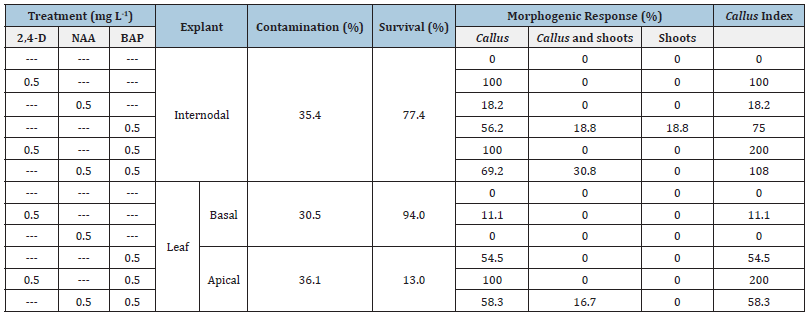
The combination of 2,4-D with BAP in equal amounts, 0.5mg L-1, performed better than the three growth regulators alone or the combination between NAA and BAP, in inducing compact light green calli on 100% of the survivor explants (Table 1). The same held true analyzing the CI values which also accounts for the quantity of calli induced (Table 1). Even though 2,4-D alone (0.5mg L-1) induced callus formation as well as its combination with 0.5mg L-1 BAP from internodal segments, calli proliferated less, as shown by CI (100 compared to 200). Additionally, 2,4-D alone could only induce callus on 11.1% of leaf segments. NAA alone showed to be weaker than 2,4-D at the same concentration in inducing callus and BAP alone or in combination with NAA was shown to induce very compact dark green callus and shoot differentiation (Figure 1A-D). Even though all calli were compact, the ones induced in the presence of 2,4-D were less rigid, easier to separate, and in higher quantity, especially with combination of BAP, than the ones induced in the presence of BAP and absence of 2,4-D. Given the higher amount of calli, the ones induced with these two substances, BAP and 2,4-D, were selected for further experiments on callus propagation.
The present study demonstrates, for the first time, that both leaf and internodal stem explants may be used for propagation of specific C. ladanifer genotypes under the influence of 0.5mg L-1 BAP in the culture medium. However, internodal segments seem to be more suitable for that purpose. Regarding this plant species, only Boukili et al. [7] report shoot induction in 50% of nodal segment explants of wild adult plants under the influence of BAP (0.2mg L-1) and IBA (0.1mg L-1), but they do not report the extent of losses by contamination and death. In our study, contamination losses were low (around 30%) and the higher shoot regeneration (organogenesis) percentage from internodal segments, near 38% under the influence of 0.5mg L-1 BAP (Table 1), is only slightly lower than the highest shoot induction response reported by Boukili et al. [7]. In addition, Boukili et al. [7] had to use an antioxidant pre-treatment step to prevent explants from browning which was not needed in this work. Furthermore, the number of internodal segments and/or of leaf segments available per shoot is at least two times higher than nodal segments. Shoot regeneration from compact calli, induced from C. creticus leaf explants using TDZ with or without NAA, is reported by Madesis et al. [10]. In the present study it is not possible to conclude if organogenesis happened from the callus mass or directly from explant original tissues (e.g., cambial meristem) because in some internodal explants shoots regenerated without a visible callus mass.
Two types of calli were induced using both types of explants. Dark green compact calli were induced using BAP (0.5mg L-1) with or without NAA (0.5mg L-1) and were associated to shoot regeneration (Figure 1B). Light green, with white and yellow areas, compact calli, less rigid and in higher amount than dark green calli were induced under the influence of 2,4-D (0.5mg L-1) with or without BAP (0.5mg L-1) (Figure 1C-E). The present study reports, also for the first time, callus induction from C. ladanifer explants even though callus formation during rooting with IBA is reported by Boukili et al. [7]. Nevertheless, Pela et al. (2000) reported callus induction from shoot-tips and lateral buds explants of C. creticus using BAP, which was enhanced when combined with indole-3-acetic acid. Zygomala et al. (2003) reported callus induction from internodal segments of C. creticus using NAA alone. Callus may find applications for mass propagation purposes, genetic or cytoplasmatic manipulation, and production of secondary metabolites in liquid cultures. Shoot multiplication, rooting and acclimatization to field conditions were not evaluated in the present study but two studies report protocols for those steps [6,7].
Callus proliferation and evaluation
Light green compact calli were obtained from internodal stem segments using 0.5mg L-1 BAP and 0.5mg L-1 2,4-D supplemented medium (Figure 1C) which was the medium that induced higher amount of callus mass in the previous experiment (Table 1). Callus proliferation was evaluated using 2,4-D and different amounts of BAP during five subcultures. The initial mass of transferred callus showed to be inversely correlated to the percentage of growth, i.e., smaller quantities of callus grew more (Table 2). Due to this feature only the three last subcultures were used for data analysis because they did not show correlation between growth and initial mass, meaning that the initial mass transferred on those subcultures was stabilized (approximately 0.5cm in Ø or 70mg).
Table 2:Correlation test between initial mass of transferred calli of C. ladanifer and the percentage of growth (% Growth, dw) of all calli lines, for the five subcultures or for the last three subcultures.
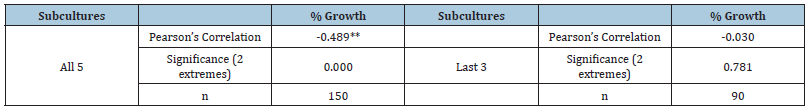
Correlation is significant for **α=0.01; *α=0.05 (n=10 x 5 or 3 repetitions).
Regarding callus proliferation, no significant difference was found between the different proportions of 2,4-D and BAP (Table 3). However, when 2,4-D was used alone, calli presented a significantly higher dry weight (Table 3). Morphologically, the three treatments continued to produce light green calli with some exceptions of uncoloured (yellow) calli, or parts of it, and some very green calli from 0.5mg L-1 2,4-D supplemented medium (Table 4) and (Figure 1E-H). However, this concentration of 2,4-D and its combination with 0.25mg L-1 BAP promoted the formation of differentiated structures erupting from calli (Figure 1G,H&J). Necrotic tissues (brown and black/oxidized parts), mostly inside de callus, appeared in calli from all treatments but more in accordance with the degree of differentiation. Differentiation degree may be related to the dry weight.
Table 3:Callus evaluation of the three callus proliferation subcultures of C. ladanifer.

In each column, different coefficient letter means statistically significant difference (α=0.05) by the post-hoc Tukey’s test. (Mean value ± standard deviation).
Table 4:Percentage of morphological evaluations of the three callus proliferation subcultures of C. ladanifer.
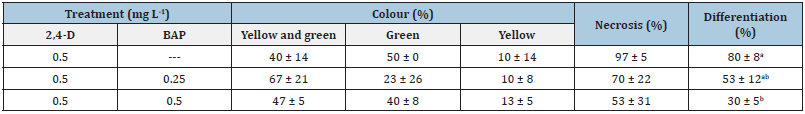
In each column, different coefficient letter means statistically significant difference (α=0.05) by the non-parametric Kruskal-Wallis test. (Mean value percentage of happening of the events ± standard deviation).
In a side experiment where calli with root-like differentiation were transferred to fresh medium containing equal amounts of 2,4- D and BAP (0.5mg L-1), some started to develop true roots (Figure 1I). Histologic observation also suggests that these differentiated structures are roots, with a defined central vascular cylinder (CVC) and a cortex (Figure 2A1-2&C1-3), although not completed perhaps because of the initial stage of development. In longitudinal sections, phloem sieve tubes with transversal cell walls are observed in the CVC periphery (better observed as green stripes under the microscope than in the photographs) and xylem tracheary-type cells are also observed in the centre (Figure 2A2). A pattern of primary xylem (metaxylem) is observed in transversal sections (Figure 2C3). In longitudinal and transversal sections of such differentiated structures, large cells are observed at the cortex. An epidermal layer was also sometimes observed (Figure 2B) and endodermis assembling the CVC may be observed in Figure 2A2. However, the root cap pattern was not well observed. Non-differentiated calli parts were also histologically examined showing, overall, no tissue organization (Figure 2E). However, a supposed non-differentiated part from a callus grown in 0.5mg L-1 2,4-D supplemented medium showed some tissue organization in what seems to be an external meristematic organization due to a line of highly stained small cells (Figure 2D1-2).
Figure 2:Histological slides photographs toluidine blue stained; A and B: Callus with differentiated structures proliferating on 0.5mg L-1 2,4-D alone (B) or with 0.5mg L-1 BAP (A) ; A1: longitudinal section of the differentiated structure (x10); A2: longitudinal section of the differentiated structure (x40), arrows pointing to sieve tubes; C: Callus from 0.5mg L-1 2,4-D + 0.5mg L-1 BAP supplemented medium, with differentiated structures (more deep slide than A and B); C1: several differentiated structures (x4); C2: longitudinal and transversal sections of several differentiated structures; C3: transversal section of a differentiated structure; D1 and D2: Callus proliferating on 0.5mg L-1 2,4-D + 0.5mg L-1 BAP supplemented medium without differentiated structures however with some degree of tissues specialization; E: Callus proliferating on 0.5mg L-1 2,4-D + 0.5mg L-1 BAP supplemented medium without differentiated structures (in colour).

Studies regarding a model plant, Arabidopsis thaliana, calli induction with auxin alone or in association with cytokinin showed that the calli initiating in the pericycle (or analogous in embryoapical organs) were very similar to root lateral meristems but not exactly the same either morphologically (e.g., lack of apical organization) or in expressed genes, with the potential to form true roots or even shoots, depending on the growth regulator and also on a time window of that meristem development [14,15]. Therefore, despite the rhizogenic capacity of calli induced with 2,4-D, it was considered relevant to evaluate the shoot organogenic capacity of these calli.
Using BAP (0.5mg L-1 and 1.0mg L-1) combined or not with NAA (0.5mg L-1) for shoot organogenesis from callus cultured on 0.5mg L-1 BAP + 0.5mg L-1 2,4-D supplemented medium, the only organogenic response was the formation of roots. Necrosis appeared in almost all calli regardless the treatment, and dead (full necrosis) happened in 30% of the calli subcultured in control medium and in 15% and 20% of the first calli subcultured in 0.5mg L-1 NAA in combination with 0.5mg L-1 or 1mg L-1 BAP supplemented medium, respectively. In this conditions, light green calli showed no potential for shoot organogenesis. In fact, those associated with shoot regeneration were the dark green calli. In sum, light green compact calli, obtained either from basal leaf or internodal stem segments under the influence of the auxin 2,4-D are rhizogenic. However, that rhizogenic ability may be reduced by increasing the amount of BAP. Using other auxin, like NAA, or BAP alone or in combination, also reduce the rhizogenic ability but do not induce shoots formation. This type of callus is not suitable for propagation purposes at the conditions tested but they may be further explored as starting material for cell and root liquid cultures for the synthesis of secondary metabolites. However, root cultures may not be suitable for the synthesis of important secondary metabolites because root cultures of C. creticus were shown to be unable to produce resin compounds such as diterpenes in contrast to what happened with shoot cultures [11].
Conclusion
Leaf and internodal stem explants from Cistus ladanifer showed to be suitable starting material, and better than nodal explants, for shoot regeneration under the influence of BAP and for callus production under the influence of 2,4-D and BAP. Two types of compact calli were obtained. Light green rhizogenic calli were obtained under the influence of 2,4-D, yet root differentiation was inhibited by including BAP in the culture media, mostly in equal amounts. Those calli showed not to be suitable for shoot regeneration under the influence of BAP and NAA. Dark green calli were induced in the same conditions as the regeneration of shoots under the influence of BAP alone with or without NAA but further studies need to be done to evaluate their organogenic ability and ultimately their utility to increase shoot multiplication rates. In addition, the ability to synthetize specific compounds with pharmacological interest should be evaluated for the two types of calli as cell cultures or even as starting material for organ (e.g., roots, shoots, trichomes) cultures.
Funding
This work was supported with National Funds by Operação CENTRO-01-0247-FEDER-033815 under the Project InovEPInovação com extratos de plantas: na senda de produtos farmacêuticos disruptivos e de base tecnológica, and by Portuguese National Funding Agency for Science, Research and Technology (FCT), under the Grant PB/DB/135330/2017 and under the project CERNAS-IPCB [UIDB/00681/2020].
References
- Frazão DF, Raimundo JR, Domingues JL, Quintela-Sabarís C, Gonçalves JC, et al. (2018) Cistus ladanifer (Cistaceae): A natural resource in Mediterranean-type ecosystems. Planta 247:289-300.
- Raimundo JR, Frazão DF, Domingues JL, Quintela-Sabarís C, Dentinho TP, et al. (2018) Neglected Mediterranean plant species are valuable resources: the example of Cistus ladanifer. Planta 248:1351-1364.
- Espinosa-Leal CA, Puente-Garza CA, García-Lara S (2018) In vitro plant tissue culture: means for production of biological active compounds. Planta 248: 1-18.
- Talavera S, Gibbs P, Herrera J (1993) Reproductive biology of Cistus ladanifer (Cistaceae). Plant Syst Evol 186:123-134.
- Dias LS, Pereira IP, Dias AS (2019) Seed germination in Cistus ladanifer: Heat shock, physical dormancy, soil temperatures and significance to natural regeneration. Plants 8(3): 63.
- Iriondo JM, Moreno C, Pérez C (1995) Micropropagation of six rockrose (Cistus) species. Hort Science 30:1080-1081.
- Boukili M, Chakir S, Haloui Z, Echchgadda G (2018) Rapid in vitro multiplication of Cistus ladanifer var. maculates Dun. J Mater Environ Sci 8:1489-1494.
- Bhatia S, Sharma K, Dahiya R, Tanmoy B (2015) Modern applications of plant biotechnology in pharmaceutical sciences. Academic Press, London, UK.
- Efferth T (2019) Biotechnology applications of plant callus cultures. Engineering 5(1): 50-59.
- Madesis P, Konstantinidou E, Tsaftaris A, Nianiou-Obeidat I (2011) Micropropagation and shoot regeneration of Cistus creticus Creticus. J Appl Pharm Sci 1: 54-58.
- Skorić M, Todorović S, Gligorijević N, Janković R, Živković S, et al. (2012) Cytotoxic activity of ethanol extracts of in vitro grown Cistus creticus creticus L. on human cancer cell lines. Ind Crops Prod 38:153-159.
- Murashige T, Skoog F (1962) A revised medium for rapid growth and bioassays with tobacco tissue cultures. Physiol Plant 15(3): 473-497.
- Wakhlu A, Barna K (1989) Callus initiation, growth and plant regeneration in Plantago ovata cv. GI-2. Plant Cell Tissue Organ Cult 17: 235-241.
- Atta R, Laurens L, Boucheron‐Dubuisson E, Guivarch A, Carnero E, et al. (2009) Pluripotency of Arabidopsis xylem pericycle underlies shoot regeneration from root and hypocotyl explants grown in vitro. Plant J 57(4): 626-644.
- Sugimoto K, Jiao Y, Meyerowitz EM (2010) Arabidopsis regeneration from multiple tissues occurs via a root development pathway. Dev Cell 18(3): 463-471.
© 2022 José Carlos Gonçalves. This is an open access article distributed under the terms of the Creative Commons Attribution License , which permits unrestricted use, distribution, and build upon your work non-commercially.
 a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in
a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in 







.jpg)






























 Editorial Board Registrations
Editorial Board Registrations Submit your Article
Submit your Article Refer a Friend
Refer a Friend Advertise With Us
Advertise With Us
.jpg)






.jpg)














.bmp)
.jpg)
.png)
.jpg)










.jpg)






.png)

.png)



.png)






