- Submissions

Full Text
Journal of Biotechnology & Bioresearch
Bacterial Infections by MRSA and VRSA and Treatment Problems: Mini Review
Monireh Rahimkhani1* and Arya Khavari Daneshvar2
1Department of Lab Medical Sciences, Faculty of Allied Medical Sciences, Tehran University of Medical Sciences, Iran
2Faculty of Engineering, University of Tehran, Iran
*Corresponding author: Monireh Rahimkhani, Associate Professor, Department of Lab Medical Sciences, Faculty of Allied Medical Sciences, Tehran University of Medical Sciences, Tehran, Iran
Submission: September 21, 2022;Published: October 13, 2022

Volume4 Issue2October , 2022
Abstract
Staphylococcus aureus is an important pathogen that causes various infections. In recent years, multidrug resistance in Staphylococcus aureus strains has been on the rise. Considering the frequency of MRSA and VRSA strains in hospitals and medical centers as well as in different communities, it seems necessary and important to observe the use of appropriate drugs to reduce antibiotic resistance and reduce the economic costs of treatment. However, new treatment options using other drugs and biological compounds apart from antibiotics for the treatment of MRSA and VRSA, are slowly emerging.
Keywords:Staphylococcus aureus; Methicillin; Antibiotic resistant; Vancomycin
Introduction
Antimicrobial resistance among Gram-positive bacteria, especially in Staphylococcus aureus, Enterococcus Faecium, Enterococcus Faecalis and Streptococcus Pneumoniae is a serious threat to public health. These microorganisms have several resistance mechanisms against antibiotics that are currently used in many clinical surgeries. The prevalence and spread of these mechanisms are significantly different depending on the microorganism [1].
Staphylococcus aureus is Gram-positive cocci that have spread worldwide and are often present on the mucous membranes and skin. 130 years ago, this bacterium was first identified as the causative agent of purulent abscesses. Staphylococcus aureus is the most important agent of hospital infections and can cause superficial or deep infections, which are sometimes fatal and cause widespread damage to the body. Staphylococcus aureus infections range from mild soft tissue and skin infections to meningitis, endocarditis, pneumonia, chronic osteomyelitis, or bacteremia, which are associated with severe complications and mortality.
The high prevalence of these bacteria in the environment and their high resistance to dry surfaces have caused them to be considered a measure of contamination in hospitals. Due to the abundant presence of staphylococcal species on the skin surface and frequent injections in hospitalized patients, the prevalence of infections caused by these bacteria in hospitalized patients is very high. These bacteria are often found on the skin and inside the nose of healthy people [2].
Staphylococci are one of the bacterial groups that have high resistance to different groups of antibiotics. Investigating antibiotic resistance helps to prevent the spread of resistant strains and also to prevent the transfer of resistant strains of antibiotics in Staphylococcus isolates. Awareness of the rate of development of resistance to several antibiotics (MDR), as well as the effective factors in creating multiple resistances, can be considered an effective method in preventing the spread of such infectious isolates. Studies show the high sensitivity and accuracy of molecular methods, including the PCR-multiplex method, in determining and tracking genes encoding antibiotic resistance.
MRSA
The outbreak of methicillin-resistant Staphylococcus aureus was investigated for the first time in 1961, and it spread all over the world from 1980 onwards. After that, in 1991, comprehensive research was conducted in England, which announced the spread of MRSA. Studies conducted in America in 1999 showed the prevalence of MRSA in blood culture samples to the extent of 26.1%. Due to the increasing incidence of this infection in 2004-2005, the prevalence of MRSA in some areas of America was reported as 49%. Studies conducted between 1997 and 1999 in hospitals around the world reported the prevalence of MRSA in Japan as 67%, 40% in South America, 35% in Latin America, 32% in America, 26% in Europe, and 23% in Australia. This issue indicates the upward trend in the increase of antibiotic resistance around the world. Similar reports were published by Totsuka in 1999 and Yamazaki in 2008. In the studies conducted in the Netherlands between 2001 and 2002, 42% of Staphylococcus aureus strains were diagnosed as MRSA. Similar studies in America in 2004-2005 reported the prevalence of MRSA in different regions between 10 and 49%. Based on a review paper in England between 1991 and 2001 it was shown that MRSA infections and their prevalence increased, So, that between the years 1999 to 2001, the prevalence of MRSA among the infections caused by Staphylococcus aureus increased from about 30% to about 45%. In similar studies in the Netherlands, the prevalence of methicillinresistant Staphylococcus aureus strains was reported as 42%. In the studies conducted in Iran, the prevalence of antibiotic resistance in Staphylococcus aureus in different cities such as Tehran, Hamedan, Shiraz, Babol, and other cities has had differences. Based on a 2001 study in Tehran, the prevalence of MRSA was reported as 33.4%. In a similar study in 2006, the prevalence of MRSA was reported to be 42% in Babol. Along with the upward trend, the resistance rate of MRSA in 2009 in Tehran city was reported as 39% [3].
Methicillin-resistant Staphylococcus aureus is responsible for a variety of antibiotic-resistant infections. This type of Staphylococcus aureus is resistant to beta-lactam antibiotics such as penicillin (methicillin-nafcillin-oxacillin) and cephalosporins, so these antibiotics have no don’t affect diseases. Although there are bacteria in the body of healthy people, resistance to antibiotics can lead to severe infections and even death. The prevalence of MRSA is higher, especially in hospital infections, patient care centers, among patients with open wounds, and prostheses, and also in patients with immune deficiency systems [4].
Methicillin-resistant Staphylococcus aureus (MRSA) is one of the main causes of hospital-acquired infections, especially in developing countries. Globally, MRSA is increasingly becoming a problem in healthcare facilities and communities [5]. Recently, hospital-acquired infections caused by MRSA affect patient care with increased morbidity, mortality, and costs due to increased length of hospital stay and the use of more expensive antimicrobial agents [6]. Undoubtedly, Staphylococcus aureus is one of the most important causes of infection at the community levels and hospital; this can be explained by its widespread distribution in nature and the environment, in addition to being a natural microbiome in the skin and the nose of some people. In the hospital, other factors play a role in its spread. Sabouni [7] has reported the most isolation of methicillin-resistant Staphylococcus aureus belongs to pediatric intensive care units in hospitals [7]. The difference in the frequency of MRSA among different researchers is definitely due to the difference in the study locations, population differences, and health indicators of those centers and communities. Also, the lack of an infection control program in some hospitals and clinics, especially in most developing countries, has a great impact on the effectiveness of bacterial transmission between patients and the hospital environment [8].
Considering that methicillin-resistant strains are only sensitive to the antibiotic vancomycin, excessive use of this antibiotic in the treatment of MRSA infections can have dangerous consequences. This problem can be caused by factors such as contamination of surgery equipment, contamination of hospital environments, as well as excessive use and inappropriate use of antibiotics. MRSA is resistant to penicillin-like beta-lactam antibiotics. However, some drugs still have activity against MRSA, including glycopeptides (such as teicoplanin and vancomycin), tigecycline, linezolid, daptomycin, and even some newer beta-lactams, such as ceftobiprole and ceftaroline. However, MRSA has shown a large spread in different environments such as hospitals, communities, and recently in animals over time. This is a challenge for infection control systems that only focus on healthcare-associated infections (HAIs). In addition, although resistance to MRSA agents usually occurs through bacterial mutation, there are reports of transmission of resistance to linezolid and glycopeptide antibiotics, which is of major concern. In a 2021 study in Nepal, among 39 S aureus isolates, all isolates were resistant to penicillin, followed by erythromycin (94.9%) and gentamicin (94.9%), and none of the MRSA strains was resistant to vancomycin [9]. In 2020 in India, 75% of MRSA isolated from inpatients and outpatients showed resistance to cotrimoxazole and erythromycin in 56.4% and 53.9%, respectively [10]. These antibiotics and mupirocin are usually used randomly to treat a generalized and severe pyogenic infection. Increasing rates of staphylococcal infections among patients and changing patterns of AMR have led to renewed interest in the use of clindamycin therapy in the treatment of such infections. The high rate of MRSA isolation and resistance to erythromycin, ampicillin, penicillin, etc. indicate that these antibiotics are ineffective against MRSA. Vancomycin seems to be an antimicrobial agent that has shown high sensitivity against Staphylococcus aureus in these studies. Hence, vancomycin may be used as the choice drug for the treatment of severe infections caused by MDR-MRSA. However, frequent monitoring of vancomycin susceptibility and routine tests should be performed both by determining the MIC and checking for the vancomycin resistance gene in the studied isolates. Regular surveillance of nosocomial infections, including MRSA and MSSA antibiogram monitoring, and establishing a definitive antibiotic policy may help reduce the incidence of MRSA infections. However, new treatment options using other drugs and biological compounds apart from antibiotics for the treatment of VRSA and MRSA, are slowly emerging [11].
A study was conducted on blood and wound samples of patients hospitalized in Tehran University of Medical Sciences hospitals. 41 MRSA isolates were obtained from these samples, 23 of which were isolated from men and 18 from women. Most MRSA was isolated from the age group between 45-60 years with 17 cases and in this study, there was no significant relationship between age groups and gender with the presence of MRSA [12]. In Mahrez et al. [12] study, 254 patients (63.2%) were male and 148 patients (36.8%) were female. There was no significant difference between the gender of patients and the prevalence of MRSA infections (P=0.09). There was no significant relationship between the average age of patients and the prevalence of MRSA infections [13]. Most of the studies have stated that there is no significant relationship between gender and age of contracting MRSA, but sometimes the studies that express a significant relationship can be attributed to the difference in the infection rate between men and women due to anatomical locations, health behaviors, environmental experiences, Stress and risk exposure occur [14]. From some of the results of the studies, the incidence of rural people was higher than that of people living in the city. This can be attributed to the difficulty of working in villages such as agriculture, lack of personal hygiene, and lack of surgical units in rural hospitals [15]. Undoubtedly, Staphylococcus aureus is one of the most important causes of infection at the hospital and community levels. This can be explained by its widespread distribution in nature and the environment, in addition to being a natural microbiome in the skin and the nose of some people. In the hospital, other factors play a role in its spread. In a study, MRSA isolates were obtained from hospitalized patients in different departments of university hospitals in Tehran, where the highest amount of MRSA was obtained from the ICU department and the lowest from the NICU and pediatric departments [16].
Resistant mechanism:Rocchettia [16] and his colleagues investigated the presence of the mec A gene in blood samples to confirm MRSA isolates and called it a reliable method to confirm MRSA isolates [17]. Most studies that have examined MRSA have used only oxacillin and cefoxitin for screening. Compared with oxacillin, cefoxitin is a better drug to detect the mec A gene in MRSA and is considered a marker. Detection of the mec A gene or its product PBP2a by cefoxitin is known the gold standard for confirming MRSA [18]. In Figure 1, an overview of the development of antibiotic resistance in Staphylococcus aureus is shown.
Figure 1: Overview of the development of resistance in staphylococci.
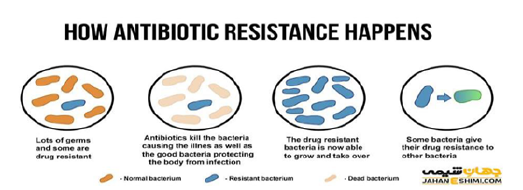
The gene responsible for methicillin resistance (mac A) is part of a mobile genetic found in all MRSA strains. Hiramatsu [18] showed that mec A is part of a genomic element named the staphylococcal caste chromosome (SCCmec). To date, four different SCCmec elements have been characterized, with different size from 21 to 67kb. Unlike the different strains of methicillin-susceptible Staphylococcus aureus (MSSA) that cause infection, only a limited number of clonal strains cause epidemics of MRSA infections. This distinction reflects the genetic limitations of the horizontal transfer of the Mec element from staphylococcal species related to Staphylococcus aureus. Studies show that the emergence of epidemic MRSA clones was partly the result of the transferring of the Mec gene to an environmentally compatible MSSA clone. A recent increase in reported community-acquired MRSA (CA-MRSA) infections in patients from different countries was associated with the diagnosis of unique SCCmec, type IV. This fragment is smaller than other fragments and seems to be more mobile from the genetic point of view and currently does not carry other antimicrobial resistance genes. It also appears to occur in a more deferent range of MSSA genetic backgrounds, indicating that it is heterologously transmitted more easily than other staphylococcal strains [19].
Methicillin resistance have the chromosomal mecA gene. MecA encodes the penicillin-binding synthase protein PBP2a, which is a 78kDa protein. PBPs are membrane-bound enzymes that catalyze the transpeptidation reaction necessary for the cross-linking of peptidoglycan chains. Their function is similar to serine proteases and they seem to have evolved from them. PBP2a replaces other PBPs and, due to its minimum affinity for all β-lactam antibiotics, enables Staphylococcus aureus to survive exposure to high concentrations of these antibiotics. S. aureus acquires resistance to anti-staphylococcal penicillin through the expression of additional penicillin-binding protein (PBP) (PBP2a). Unlike other PBPs, PBP2a is resistant to the inhibitory effects of all β-lactams (except ceftobiprole and ceftaroline) and is always encoded by the auxiliary mecA gene. MecA expression is controlled by a signal-inducing protein and a repressor located in the mecA operon. Recently, mec alternative alleles have also been described, for example, mecC has 70% nucleotide identity with mecA and is commonly found in MRSA isolated from cattle (LA-MRSA). MecC-MRSA appears to have a minimal inhibitory concentration (MIC) of oxacillin due to the distinct characteristics of the PBP2a homolog (PBP2c), including a higher binding affinity for oxacillin than cefoxitin and sensitivity to β-penicillin. Lactam inhibitor compounds.
Accordingly, mecC-MRSA was successfully treated with betalactams in an experimental model of endocarditis. Rare homologs of Mec are found in other strains of Staphylococcus such as mecA1 in Staphylococcus Sciurid, MecD in Macrococcus spp and mecA2 or MecB in Staphylococcus Vituline. Based on genomic studies, it is hypothesized that mecA was acquired several years before the first diagnosis of MRSA in 1961. Molecular tests have simplified the rapid diagnosis of MRSA in clinical samples. However, the correlation between the presence of mecC or mecA and phenotypic resistance to oxacillin is not absolute. Approximately 3% of S. aureus containing mecA are phenotypically susceptible to oxacillin. As mentioned above, this expression has previously been explained by heterogeneous synthesis of PBP2a, but a genomic study provided a fascinating alternative mechanism. By examining two clinical isolates of methicillin-susceptible Staphylococcus aureus positive for MecA, it was shown that mecA expression was suppressed by gene disruption through IS1181 insertion in one case and MecA frameshift mutation in the other case. A recent study by Chung et al. [19] showed that strains of MRSA were sensitive to clavulanic acid/penicillin combinations treatment. The genomic basis of this phenomenon is due to the connection of mutations in the promoter and coding sequences of mecA [20].
Therefore, resistance to methicillin causes resistance to all beta-lactam agents, including cephalosporins. New research have determined the pure crystal structure of a soluble derivative of PBP2a, which differs from other PBPs, and its active site blocks the binding of all β-lactams but allows to proceed the transpeptidation reaction [21].
The expression of methicillin resistance in strain of Staphylococcus aurous is variable. In some MRSA strains, it is regulated by homologs of the blaZ regulatory gene. These genes include MecI and MecR1, which adjust MecA’s response to β-lactam antibiotics like the regulation of blaZ by blaR1 and blaI genes in exposure to penicillin. The DNA sequences bound by inhibitor genes are similar to inhibit gene activation.
As previously mentioned, the mecA gene is always part of a larger mobile and unique genetic element. Since there is no mecA homolog in MSSA, it is assumed that mecA is derived from one of several other coagulase-negative staphylococcal species. Coto et al. identified a mecA gene in a methicillin-susceptible S. sciuri with 88% amino acid identity to Methicillin resistant Staphylococcus aureus [22].
Staphylococcal resistance to penicillin is regulated by blaZ, the gene encoding beta-lactamase. This enzyme is mainly synthesized extracellularly when staphylococci are exposed to beta-lactam antibiotics, hydrolyzes the beta-lactam ring, and deactivates the beta-lactam. BlaZ is regulated and control by two regulatory genes, anti-repressor blaR1 and repressor blaI. Recently researches have shown that the signaling pathway responsible for betalactamase synthesis requires the sequential cleavage of regulatory proteins BlaR1 and BlaI. Further exposure to β-lactams, BlaR1, a transmembrane sensor transducer, cleaves itself. Lakhundia and Zhang [22] have shown that the cleaved protein acts as a protease that directly or indirectly cleaves the BlaI repressor and allows blaZ to synthesize the enzyme [23]
Figure 2: The pathway of cell damage and disintegration.
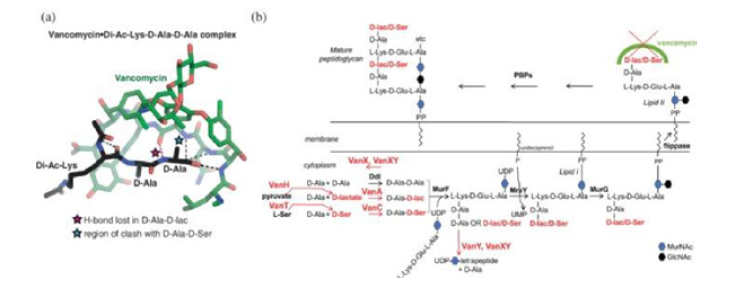
Drug-resistant Gram-positive bacteria such as Staphylococcus aureus, Clostridium difficile, Streptococcus pneumoniae, and Enterococcus faecium are considered by the Centers for Disease Control and the WHO as urgent or serious threats. The primary glycopeptide of vancomycin is a natural product produced by Actinobacteria Amycolatopsis orientalis that was discovered in the 1950s by researchers at Eli Lilly (Indianapolis) and has been in clinical use since 1958. Industrial efforts have led to the development of next-generation glycopeptides or their lipid analogs, lipoglycopeptides, including telavancin, dalbavancin, and oritavancin. The influence mechanism of vancomycin and other glycopeptide antibiotics is by binding to the terminal d-Ala-d-Alaof N-Acetylmuramol-N-acetylglucosamine. This binding inhibits the activity of penicillin-binding proteins PBPs to cross-link lipid II to mature peptidoglycan, thus compromising the integrity of the cell membrane and leading to osmotic stress and cell bursting (Figure 2).
A significant increase in the use of vancomycin to treat infections caused by methicillin-resistant staphylococci (both coagulasepositive and negative), Clostridium Difficile and enterococcal infections caused the emergence of vancomycin-resistant staphylococci. Staphylococcal resistance to vancomycin in a clinical isolate was reported for the first time in a strain of Staphylococcus hemolyticus. In 1997, the first report of vancomycin-resistant S. aureus (VISA) was from Japan, and subsequent cases were subsequently reported from other countries. VISA strains were all MRSA and were not clonal. Many of these patients had MRSA infections and had received vancomycin during treatment.
Vancomycin and related glycopeptides are the last resort for the treatment of severe infections caused by Gram-positive bacteria such as Enterococcus species, Staphylococcus aureus and Clostridium difficile. For a long time, vancomycin was an effective antibiotic due to its antibacterial activity based on binding to the bacterial cell envelope instead of a protein target, which is the case with most antibiotics, and there was no resistance to it. 30 years after the initial discovery of resistance to vancomycin, with extensive research, significant progress has been made on the molecular mechanisms of enzymatic resistance. These molecular data can accelerate inhibitor discovery and optimization efforts to reverse vancomycin resistance [24]. Two recent reports of infections caused by vancomycin-resistant Staphylococcus aureus (VRSA) are of great concern because they reflect both complete resistance and a different mechanism of dissemination. Unlike the chromosomal resistance of VISA strains, VRSA strains acquire resistance by transferring the VanA operon from Enterococcus faecalis.
Resistant mechanism: Two forms of resistance of Staphylococcus
aureus to vancomycin have been identified.
a) A form has been identified in VISA strains with vancomycin
MICs of 8-16μg/ml. Vancomycin-resistant strains remain sensitive
but have resistant subpopulations. It is assumed that when exposed
to vancomycin, VISA strains are selected from vancomycin-resistant
subpopulations. Decreased susceptibility to vancomycin appears to
be due to changes in peptidoglycan biosynthesis. VISA strains are
notable for the excessive amounts of synthesized peptidoglycan
that result in irregular shape and thickened cell walls. Also, crosslinking
of peptidoglycan strands decreases and more D-Ala-D-Ala
residues are available to bind and trap vancomycin (Figure 3).
The bound vancomycin then acts as a barrier for drug molecules
to reach their target on the cytoplasmic membrane. The molecular
mechanisms for these changes in peptidoglycan biosynthesis are
not known and there is not much information.
Figure 3: Resistance based on d-Ala-d-lac types Van A, Van B, Van D, Van F and Van M.
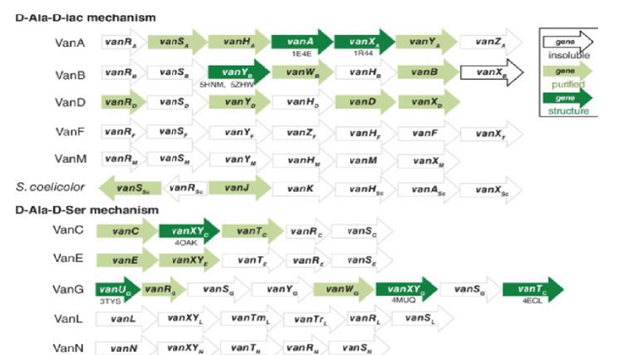
b) The second form of vancomycin resistance results from
the possible transfer of the VanA operon from a vancomycinresistant
E Faecalis. These VRSA strains showed complete
resistance to vancomycin with MIC≥128μg/ml. Resistance in these
isolates is caused by changing the terminal peptide to D-Ala-D-Lac
instead of D-Ala-D-Ala. Synthesis of D-Ala-D-Lac occurs only upon
exposure to low concentrations of vancomycin. The environmental
compatibility between Staphylococcus aureus and enterococcus
increases the probability of plasmid exchange between them, and
due to the increased probability of colonization of patients with
MRSA and vancomycin-resistant enterococci, the resistance of
these strains to beta-lactams and glycopeptides also increases. It is
predicted that VRSA strains are going to spread more rapidly in the
future [25].
c) From a molecular point of view, vancomycin resistance in
Staphylococcus aureus can be caused by acquiring the vancomycin
resistance determinant VanA, or usually through combinations
of mechanisms independent of VanA, mainly mutations in genes
involved in cell wall biosynthesis. VanA-mediated resistance is
associated with high-level resistance (VRSA, with a vancomycin
MIC of 16mg/L and higher) and is due to the acquisition of the VanA
operon, located in the Tn1546 transposon, which is most associated
with vancomycin. Resistance in enterococci was first described
in 2002 in a patient with end-stage renal failure and diabetic foot
infection. Subsequent molecular studies revealed that the VRSA
isolates a harbored plasmid that had acquired Tn1546 from a
vancomycin-resistant Enterococcus Faecalis (Figure 4). While this
report and previous experimental work have raised concerns about
the spread of high-level vancomycin resistance. Although most
VRSA strains to date belong to clonal complex 5, genomic analysis
of 12VRSA strains from the United States revealed that they are
genetically unrelated to the last common ancestor around 1960,
possibly as a result of acquiring a plasmid containing VanA operon
Figure 4:Transfer of Tn1546 from a VRE plasmid to a staphylococcal resident plasmid.
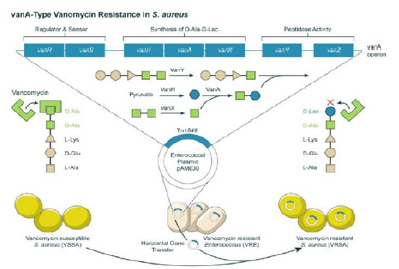
Phenotypically, these strains have low-level vancomycin resistance (VISA vancomycin MIC 4.8mg) or may not be resistant when tested by conventional methods, while having heterogeneous resistance to hVISA vancomycin. They are also characterized by thickened cell walls, slower growth, or increased autolysis. The molecular basis of these changes is complex and multigenic. Most of the mutations involve regulators of cell wall biosynthesis, such as the two-component regulators vraRS, graRS, and walKR. However, the mutation in the rpoB gene can also be associated with the VISA phenotype. Despite the different genetic resistance mechanisms and phenotypes of VRSA and VISA, these have common features that distinguish them from MRSA. Unlike MRSA, VISA and VRSA are generally polyclonal and no significant release has been seen. To date, resistance to vancomycin has occurred secondarily during the treatment of complicated Staphylococcus aureus infections. Thus, prevention of this Resistance is likely through optimizing the management of complex MRSA infections (including appropriate source control) Implementation of antibiotic surveillance, rather than infection control, is best achieved [26]. Suitable alternatives to vancomycin include a combination of high-dose daptomycin with another antibiotic such as gentamicin, rifampin, linezolid, trimethoprim-sulfamethoxazole (TMP-SMX), or a beta-lactam. If a decrease in sensitivity to daptomycin and vancomycin is observed, the combined or individual use of the following items is recommended; Quinopristin-Dalfopristin, TMP-SMX, linezolid, or telavancin [27]. Daptomycin is a cyclic lipopeptide active against multidrug-resistant Gram-positive organisms, including methicillin-resistant Staphylococcus aureus (MRSA) and Staphylococcus aureus with reduced susceptibility to vancomycin (VISA). This drug is 4-8 times more active than vancomycin against methicillin-sensitive Staphylococcus aureus (MSSA) and MRSA and maintains most of this activity against Staphylococcus aureus by reducing vancomycin sensitivity. The mechanism of daptomycin is not fully understood. Daptomycin binds to the bacterial cytoplasmic membrane and leads to depolarization due to the loss of potassium ions from the cytoplasm. Various mechanisms have been proposed to explain daptomycin resistance, most of which are related to changes in cell wall composition, load, and fluidity. MprF mutations, which lead to increased production of Lysyl phosphatidylglycerol, and rpoB and rpoC mutations of the rpo genes for RNA polymerase subunits, have been proposed to be associated with daptomycin resistance. Treatment failures with daptomycin have been seen when administered at low concentrations [27].
References
- Chotai S, Wright PW, Hale AT, Jones WA, McGirt MJ, et al. (2017) Does intrawound vancomycin during spine surgery create vancomycin resistant organism? Neurosurgery 80(5): 746-753.
- Rahimkhani M, Mordadi A, Karami P, Zarei O (2021) Prevalence and expression of genes of type II antitoxin toxin systems in clinical isolates of methicillin-resistant Staphylococcus aureus. Journal of Pharmaceutical Research International 33(41A): 96-105.
- Dulon M, Haamann F, Peters C, Schablon A, Nienhaus A (2011) MRSA prevalence in European healthcare settings: A review. BMC Infect Dis 11: 138.
- Boucher HW, Corey GR (2008) Epidemiology of methicillin-resistant Staphylococcus aureus. Clin Infect Dis 46(suppl 5): S344-S349.
- Darboe S, Dobreniecki S, Jarju S, Jallow M, Mohammed NI, at el. (2019) Prevalence of Panton-Valentine Leukocidin (PVL) and antimicrobial resistance in community-acquired clinical Staphylococcus aureus in an urban Gambian hospital: A 11-year period retrospective pilot study. Front Cell Inf Microbiol 22(9): 1-7.
- Feßler AT, Schünemann R, Kadlec K, Hensel V, Brombach J, at el. (2018) Methicillin-resistant Staphylococcus aureus (MRSA) and methicillin-resistant Staphylococcus pseudintermedius (MRSP) among employees and in the environment of a small animal hospital. Vet Microbiol 221: 153-158.
- Sabouni FM, Bahador S, Pourakbari A, Sadeghi B, Hosseinpour SR, et al. (2014) Virulence factors of Staphylococcus aureus isolates in an Iranian referral children's hospital. Osong Public Health Res Perspect 5(2): 96-100.
- Pandey S, Raza M, Bhatta C (2012) Prevalence and antibiotic sensitivity pattern of methicillin-resistant-Staphylococcus aureus in Kathmandu medical college-teaching hospital. Journal of Institute of Medicine Nepal 34(1): 13-17.
- Dhungel S, Rijal KR, Bindeshwar Y, Binod D, Nabaraj A, at al. (2021) Methicillin-Resistant Staphylococcus aureus (MRSA): Prevalence, antimicrobial susceptibility pattern, and detection of mec A gene among cardiac patients from a tertiary care heart center in Kathmandu, Nepal. Infect Dis (Auckl) 14: 11786337211037355.
- Gurung RR, Maharjan P, Chhetri GG (2020) Antibiotic resistance pattern of Staphylococcus aureus with reference to MRSA isolates from pediatric patients. Future Sci OA 6(4): FS0464.
- Rahimkhani M, Mordadi A, Varmazyar S, Tavakoli A (2014) Evaluation of urinary interleukin-8 levels in patients with spinal cord injury. Recent Patents on Anti Infective Drug Discovery 9(2): 144-149.
- Mohrez M, Junidi N, Rasoulinejad M, Broumand MA, et al. (2003) Prevalence of methicillin resistant Staphylococcus aureus infections by MIC determination method in Imam Hospital. TUMJ 61(3): 182-192.s
- Gopal G, Michalczyk P, Nayeem N, Walker G, Wigmore L (2007) Prevalence and risk factors for meticillin resistant Staphylococcus aureus in adult emergency admissions a case for screening all patients? J Hosp Infect 66(1): 15-21.
- Oladeinde BH, Omoregie R, Olley M, Anunibe JA, Onifade AA (2013) A 5-year surveillance of wound infections at a rural tertiary hospital in Nigeria. Afr Health Sci 13(2): 351-356.
- Rahimkhani M, Mordadi A (2022) Survey of the lethal effect of ciprofloxacin and supernatant isolated from Staphylococcus aureus under the stress of ciprofloxacin on methicillin-resistant Staphylococcus aureus strains isolated from clinical specimens. Journal of Payavard Salamat 15(6): 578-584.
- Rocchettia TT, Martinsa BK, Faccioli PY, Oliverac NA, Mondellid NA, et al. (2018) Detection of the mecA gene and identification of Staphylococcus directly from blood culture bottles by multiplex polymerase chain reaction. Braz J Inf Dis 22(2): 99-105.
- Koosha RZ, Fooladi AA, Hosseini HM, Aghdam EM (2014) Prevalence of exfoliative toxin A and B genes in Staphylococcus aureus isolated from clinical specimens. J Inf Pub Health 7(3): 177-185.
- Hiramatsu K , Katayama Y, Yuzawa H, Ito T (2002) Molecular genetics of methicillin-resistant Staphylococcus aureus. Int J Med Microbiol 292(2): 67-74.
- Chung M, Kim KC, Conceição T, Sousa MAD, Lencastre HD, et al. (2016) Heterogeneous oxacillin-resistant phenotypes and production of PBP2A by oxacillin-susceptible/mecA-positive MRSA strains from Africa. J Antimicrob Chemother 71(10): 2804-2809.
- Dibah S, Arzanlou M, Jannati E, Shapouri R (2014) Prevalence and antimicrobial resistance pattern of methicillin resistant Staphylococcus aureus (MRSA) strains isolated from clinical specimens in Ardabil, Iran. Iran J Microbiol 6(3): 163-168.
- Pérez JR, Rosa LZ, Sánchez AG, Salcedo JHDM, Rodríguez JMA, et al. (2021) Multiple antimicrobial resistance in methicillin-resistant staphylococcus sciuri group isolates from wild ungulates in Spain. Antibiotics (Basel) 10(8): 920.
- Lakhundia S, Zhang K (2018) Methicillin-resistant Staphylococcus aureus: Molecular characterization, evolution, and epidemiology. Clin Microbiol Rev 31(4): e00020-18.
- McGuinness WA, Malachowa N, DeLeo FR (2017) Vancomycin resistance in Staphylococcus aureus. Yale J Biol Med 90(2): 269-281.
- Cong Y, Yang S, Rao X (2020) Vancomycin resistant Staphylococcus aureus infections: A review of case updating and clinical features. J Adv Res 21: 169-176.
- Fasihi Y, Saffari F, Mansouri S, Neyestanaki DK (2018) The emergence of vancomycin-resistant Staphylococcus aureus in an intensive care unit in Kerman, Iran. Wien Med Wochenschr 168(3-4): 85-88.
- Carpenter CF, Chambers HF (2004) Daptomycin: Another novel agent for treating infections due to drug-resistant gram positive pathogens. Clin Infect Dis 38(7): 994-1000.
- Tong SYC, Eichenberger E, Holland TL, Fowler VG, et al. (2015) Staphylococcus aureus infections: Epidemiology, pathophysiology, clinical manifestations, and management. Clin Microbiol Rev 28(3): 603-661.
© 2022 Monireh Rahimkhani. This is an open access article distributed under the terms of the Creative Commons Attribution License , which permits unrestricted use, distribution, and build upon your work non-commercially.
 a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in
a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in 







.jpg)






























 Editorial Board Registrations
Editorial Board Registrations Submit your Article
Submit your Article Refer a Friend
Refer a Friend Advertise With Us
Advertise With Us
.jpg)






.jpg)














.bmp)
.jpg)
.png)
.jpg)










.jpg)






.png)

.png)



.png)






