- Submissions

Full Text
Journal of Biotechnology & Bioresearch
Optimum γ- Irradiation Dose for The Highest Biological Nitrogen Fixation of Bradyrhizobium
Alsaedi SA*, Fadhel AS, Yousir Sh A and Abdulrazaq IB
Agricultural Research Directorate, Ministry of Science and Technology, Baghdad, Iraq Introduction
*Corresponding author: Alsaedi SA, Agricultural Research Directorate, Ministry of Science and Technology, Baghdad, Iraq
Submission: May 05, 2022;Published: June 20, 2022

Volume4 Issue1June, 2022
Abstract
This study was conducted to evaluate the possibility of increasing Bradyrhizobium efficiency in fixing biological nitrogen in soil. Bradyrhizobium isolates were obtained from cowpea field in certain Iraqi provinces. Isolates were purified, propagated and authenticated. Isolates were then subjected to a range of Gamma ray dose of irradiation of 0 to 800Gy. For all isolates number of nodules formed by all isolates increased with the increase of Irradiation dose to 400Gy after which numbers of nodules of all isolates were markedly reduced. Highest nodules numbers of all isolates were observed under 400GY irradiation dose. Under the same irradiation dose of 400GY numbers of nodules differ with different isolates being the highest under isolate No. 2.
Introduction
BNF is a natural process in legume crops, where atmospheric dinitrogen (N2) is fixed into ammonia (NH3) in plant root nodules by a symbiotic form of Rhizobia, a Gram-negative Proteobacteria. It depends on legume genotype as well as Rhizobia strain, but it also reflects the interaction between crop growth and plant-available N in the soil [1].
The expanded interest in ecology has drawn attention to the fact that BNF is ecologically safe and that it’s greater exploitation can reduce the use of fossil fuels and can be helpful in reforestation and in restoration of misused lands to productivity [2,3]. At present, BNF is of great practical importance because the use of nitrogenous fertilizers has resulted in unacceptable levels of water pollution (increasing concentrations of toxic nitrates in drinking water supplies) and the eutrophication of lakes and rivers [3-5]. While BNF may be almost sufficient to the needs of the organism, fertilizer is usually applied in a few large doses, up to 50% of which may be leached [3]. This wastes energy leads to serious pollution problems, particularly in water supplies. Accordingly, there has been considerable investment in improving the capacity of legume plants for fixing atmospheric nitrogen [6-8]. This includes development of complex inoculant formulations designed to improve biological nitrogen fixation, and overall plant productivity [9].
Gamma Irradiation of legume plants as a host and/or Rhizobia were evaluated to improve the symbiosis through the effects on nodules referred to the first steps mechanism of nodule formation, as well as, inducing re-combinations in bacterial strains through conjugation to be selecting the efficient one in symbiosis to improve nodulation process. Gamma (γ) radiation has a narrow range of length high energy penetration power resulting from the nuclear disintegration of certain radioactive substances such as the isotopes Cobalt 60 (6o Co) and Cesium 137 (137Cs).
Effects of radiation are accumulative [10]. Beter N -fixing symbiosis may be brought by manipulating both rhizobia and plant hosts and by eventually creating an artificial rhizosphere. Numerous studies were conducted to induce genetic variations in Bradyrhizobium isolates to select the one of best symbiosis through alteration the expression of nodulin genes using gamma irradiation.
Therefore, this study was conducted to determine dose response curve of Gram-positive Bradyrhizobia isolates obtained from cowpea root in the field of Iraqi southern provinces. Gamma irradiation dose response curve of selected isolates will be utilized to determine the optimum Gamma Irradiation dose for the most efficient BNF of Bradyrhizobium isolate.
Methods
Collection of nodules
Cowpea root nodules were collected from different field sites in governorates in Iraq, belonging to the most important cowpea production area in Iraq (Basrah, Mysan and Al Anbar). Nodules were obtained and transferred into nodules collection vials as it was described by [11]. The vials were labeled and were stored at 4±1 oC for short-term storage.
Bacterial isolates
Nodules were surface sterilized in series of surfactants in laminar air flow cabinet by immersing in 95% v/v ethanol for 10 seconds. Followed by 30% v/v solution of sodium hypochlorite for 4min and then rinsed with sterile water. After that, they were placed in 3% v/v hydrogen peroxide followed by 5 times rinse in sterile water. The sterilized nodules were crashed with sterilized glass rod putting 1ml sterilized distilled water in petri-dish. Furthermore, the nodule suspension was streak inoculated on Yeast Mannitol Agar (YMA) [12] and incubated at 28 oC. Single colonies were selected and streaked onto YMA slant and kept at 4±1 oC for short term storage with subculturing every 4 month. Long term storage was made by storing the culture broth in 10% glycerol at -20 oC [13].
Culture of cowpea rhizobium on YMA-CR media
and shape and size of bacteria were observed for all isolates.vity, indicates that the rumen has a pH (H2O)The rumen is a sac-like chamber and is the main part where the entire fermentation process of ruminants takes place. It is very voluminous and houses a microbial population of different microbes that have evolved over the years along with their host. The environment of the rumen can be described as low in oxygen. Anaerobic microbes perform the necessary biochemical reactions for energy production, while facultative aerobes are present to remove any oxygen that may have passed over to maintain anaerobic conditions. In addition, the absence of gastric acid, along with microbial activity, indicates that the rumen has a pH (H2O) between 6 and 7 [3].
Authentication of isolates by infection tests
Isolates obtained from the cowpea root nodules were authenticated as the cowpea rhizobium, the microsymbiont of cowpea. That is by examining their ability to infect cowpea roots to stimulate the root nodulation. Infection test was performed in sterile sand soil [14,15]. Sand was autoclaved for 3 times at 15 lbs (121 oC) for 20 minutes. Sterilized seeds of cowpea local cultivars were inoculated with 8 days old liquid of different bacterial isolates inocula prepared on Yeast Extract Mannitol Broth (YMB). The inoculated and uninoculated cowpea plants were grown for 4-5 weeks and examined the nodulation on their roots in both inoculated and uninoculated plants.ria found belong to the phyla Bacteroidetes and Firmicutes [9].
Yeast manitol agar-bromothymol blue (YMA-BTB) reaction
All authenticated cowpea rhizobia cells, single bacterial colonies from the pure culture of all isolates were streaked on the plates containing YMA with 2.5ml per liter of 1.0% alcoholic bromothymol blue (YMA-BTB). Then, the plates were labeled and incubated at 28 oC. The authenticity of each isolate was detected for 10 days incubation period, with observation for color of the dye indicator on the plates [12]. Fast growing rhizobia changed color of dye indicator to yellow while slow growing rhizobia turned the dye indicator to blue.
Preparation of liquid cowpea rhizobium inocula
Cowpea Rhizobium isolates was maintained in liquid medium i.e., Yeast Extract Mannitol Broth (YMB) for inoculation on cowpea. YMB was prepared according to Vincent [16]. Five ml of YMB was dispensed on conical flasks and sealed with aluminum foil and autoclaved. The autoclaved YMB flasks were then inoculated with cowpea rhizobium isolates, maintaining two replicates for each isolate. The cowpea rhizobium colonies from the YMA slants were transferred to the YMB flasks by sterilized inoculation lop. All activities were performed in laminar air flow cabinet. The inoculated YMB flasks were then incubated at 28 oC for 7 days in horizontal shaker with 120rpm of rotation frequency.
Gamma irradiation of cowpea rhizobium isolates
This part of the work has been done in March 2015, at the laboratories of Soil and fertility department-IAEA / Vienna, Austria in Siebersdorf. The wild type of cowpea rhizobium isolate was inoculated into 40ml YMB until turbidity (OD600) reached 0.15 approximately (107CFUs/ml). The bacterial suspensions were then irradiated at 0, 50, 200, 400 and 800Gy respectively. Irradiation was done at IAEA laboratories (Seibersdorf), using a cobalt -60 source (Dose rate 2Gy/Sec.).
Mutant/s efficiency evaluation
Soil sand mixture was Autoclaved for 30 minutes at 121 oC. Five seeds, previously one hour soaked in water, of cowpea were planted in each pot. 100mL of specified inoculant were added per pot. Each inoculant was replicated 3 times in randomized complete block design. After emergence plants were thinned to just 2 plants per pot. Phosphorus in 10mg per Kg soil sand mixture was added to each pot. Plants were grown for six weeks in a green house at 20±1 oC day temperature and 16±1 oC for 10 hours night.
p>Preparation of bacterial cell suspensionSuspension was prepared using Yeast Extract Mannitol Broth (YMB). Five hundred milliliters of this broth were prepared and autoclaved for 20 minutes at 121 oC. Colonies from each irradiated isolates with (0, 50, 200, 400 and 800Gy) were incubated on Agar for 24hr. of these inoculants enough were transferred to Yeast Extract Mannitol Broth (YMB) and incubated for 5 days in shaker incubator at 28 oC until growth reached the log phase at which turbidity at OD600 was 0.451. At this turbidity level there was approximately 6.0x108 8CFUs/ml.
Plant harvesting
Plant root of each treatment were obtained by removing soil particle by light stream of water. Water on Plant roots was removed by soft tissue and number of nodules was calculated.
Results and Discussion
Authentication test
The cowpea rhizobium bacterial isolates isolated from 3 Governorates in Iraq, were tested to identify their nodulation ability. Isolated bacteria were authenticated in nodulating cowpea, their original host plant, confirming their symbiotic status.
Bromothymol blue reaction
Bromothymol blue technique is used in bacteriology as a pH indicator in the agar, it changes to yellow in case of acid production or changes to deep blue in case of alkalization. This colorant is essentially used to differentiate Rhizobia, especially Bradyrhizobium.which is Alkaline in reaction compared to Rhizobium isolates which is Acidic reaction [17]. The study showed colonies reaction of all the cowpea rhizobia on Bromothymol Blue (BTB) agar plates are slow growing group (Bradyrhizobium spp.)
Colony characteristics and growth response on YMA-CR medium
The bacterial colonies grown in YMA-CR plates were creamy white to translucent watery, smooth, circular and rose. The bacterial colonies did not absorb red color of Congo red when cultured in dark, which is the characteristic feature of bradyrhizobial colonies [18].
Soils of Siebersdorf
Soils of Siebersdorf (Table 1) used in this study is sandy clay loam soil with organic matter content in the range 5-5.2%. Total nitrogen content is 2.27% with 24.80mgKg-1 soil available P. According to these characteristics the soil is of good fertility level [19]. Adequate N and high plant available P will make this soil is appropriate for BNF process. The soil is also rich in Cation’s content due to its high CEC.
Table 1:Characteristics of soil from Siebersdorf/ Austria used in the study.
Effect of gamma irradiation on cowpea isolates
Most efficient isolates of Bradyrhizobium subjected to a range of γ-irradiation for inducing genetic variability. Isolates were initially grown in broth then subjected to assigned dose of γ-radiation. Irradiated isolates were transferred to YMA media for propagation. Cowpea seeds were inoculated with each irradiated isolate.
Effect of γ radiation on number of nodules
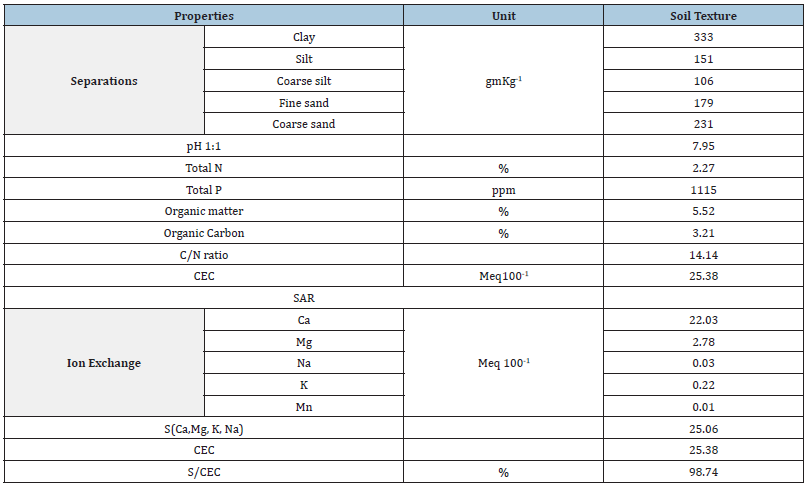
Response of three isolates of Bradirhizobium to given dose range of Gamma Irradiation of 0 to 800Gy was given in Figures 1-3. Isolates response was measured by the number of nodules found on plant root.
Figure 1:Response of Bradyrhizobium isolate 1 to a range of γ ray dose.
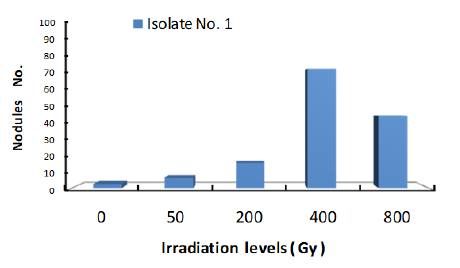
Figure 2:Response of Bradyrhizobium isolate 2 to a range of γ ray dose.
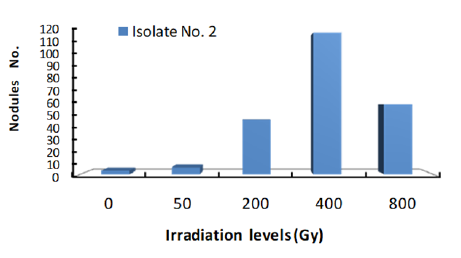
Figure 3: Response of Bradyrhizobium isolate 3 to a range of γ ray dose.
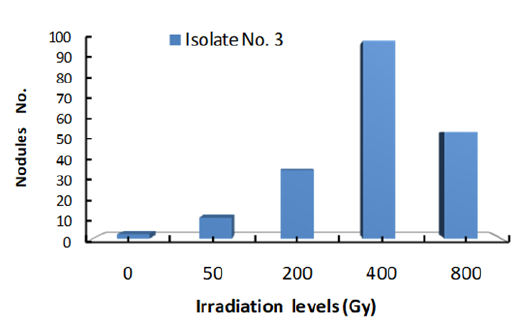
The data clearly showed that number of nodules formed by each isolate was increased with the increase of gamma radiation dose to 400Gy. Accordingly, Gamma dose of 400Gy is the optimum level of Irradiation dose for the most efficient nodulation of the three isolates. At 800Gy dose, number of nodules were markedly reduced under all three isolates which may indicate that this dose of Gamma irradiation is high enough to cause serious damage to isolate cells. These results are in agreement with those of [20-22] who had reported that Gamma Irradiation.
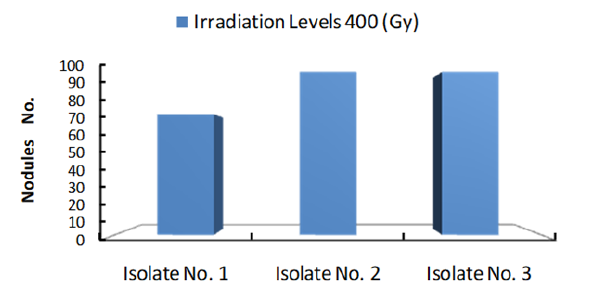
Figure 4 shows number of colonies formed by each isolate subjected to 400Gy dose of Gamma ray. The results clearly showed that number of nodules at the same radiation dose differ with different Isolates. Obviously, Isolate no. 2 showed the highest number of nodules which may indicate that this isolate under the given experimental condition is the most efficient isolate. Accordingly, one may conclude that isolates nodulation efficiency increase with the increase of radiation dose to certain limit after which nodules number will decline. Furthermore, at same irradiation dose numbers of nodules differ with different isolates.
Figure 4: Number of Cowpea root nodules formed by 3 Bradyrhizobium isolate irradiated with 400Gy γ radiation.
Conclusion
The various studies conducted in the rumen of these herbivores suggest that they are a potential source of various CAZymes, and with advanced technologies, the chance of discovering new CAZyme classes with high efficacy has increased. Further computational design and genetic engineering approaches will be beneficial in producing novel CAZymes with high activity and stability to various environmental conditions. Therefore, a comprehensive study of microflora and CAZyme profiles, including those of fungi, may be a future prospect in this field.
Acknowledgement
The authors would like to thank the Department of Microbiology, Sikkim University, for providing the computational infrastructure and central library facilities for procuring references and plagiarism analysis (URKUND: Plagiarism Detection Software).
References
- Jensen ES, Peoples MB, Hauggaard-Nielsen H (2009) Faba bean in cropping systems. Field Crops Research 115(3): 203-216.
- Burris RH (1994) Biological nitrogen fixation-past and future. In: Hegazi NA, Fayez M, Monib M (Eds.), Nitrogen fixation with nonlegumes, The American University in Cairo Press, Cairo, Egypt, 1-11.
- Sprent JI, P Sprent (1990) Nitrogen fixing organisms. Pure and applied aspects. Chapman & Hall, London, United Kingdom.
- Al-Sherif EM (1998) Ecological studies on the flora of some aquatic systems in Beni-Suef district. Cairo University (Beni-Suef Branch), Beni-Suef, Egypt.
- Dixon ROD, CT Wheeler (1986) Nitrogen fixation in plants. Thomson Science and Professional.
- Buttery BR, Park SJ (1993) Characterization of some non-fixing mutants of common bean (Phaseolus vulgaris L.). Canadian J. Plant Science 73: 977-983.
- Hardarson G (1993) Methods for enhancing symbiotic nitrogen fixation. Plant and Soil 152: 1-17.
- Devi MJ, Sinclair TR, Beebe S, Rao IM (2013) Comparison of common bean (Phaseolus vulgaris L.) genotypes for nitrogen fixation tolerance to soil drying. Plant and Soil 364: 29-37.
- Noel T, Parker R, Ring SG (1996) A comparative study of the dielectric relaxation behavior of glucose, maltose and their mixtures with water in the liquid and glassy states. Carbohydrate Research 282(2): 193-206.
- Smith-Keary PF (1975) Mutation. Genetic structure and function, Macmillan press LTD, Basingstoke, London, pp: 153-177.
- Al-Saedi SA, Razaq IB, Ali NA (2016) Utilization of 15N dilution analysis for measuring efficiency of biological nitrogen fixation under soil salinity stress. Journal of Pharmacy and Pharmaceutical Sciences 5(1): 1468-1479.
- Somasegaran P, Hoben HJ (1994) Handbook for rhizobia. Methods in Legume-Rhizobium Technology. Springer-Verlag, New York, USA, pp. 332-341.
- Tatiana KW, Ibrahima N, Serge B, Benoit S, Philippe de L, et al. (2003) Diversity of indigeneous bradyrhizobia associated with three cowpea cultivars (Vigna unguiculata (L.)) grown under limited and favorable water conditions in Senegal (West Africa). African Journal of Biology 2(1): 13-23.
- Namraj DR (2005) Study on effectiveness of native bradyrhizobial isolates for N2-fixation in soybean (Glycine max (L.)). Central Department of Botany Tribhuvan University Kathmandu, Nepal.
- Berns AE, Philipp H, Narres HD, Burauel P, Vereecken H, et al. (2008) Effect of gamma-sterilization and autoclaving on soil organic matter structure as studied by solid state NMR, UV and fluorescence spectroscopy. European Journal of Soil Science 59(3): 540-55.
- Vincent JM (1970) A manual for the practical study of the root nodule bacteria. International Biological Program, Blackwell Scientific Publications, New Jersey, USA.
- Jacinta K, Muthuri (2014) Symbiotic effectiveness of bradyrhizobium japonicum USDA 110 and sinorhizobium FREDII USDA 191 on two different soybean cultiv ARS. European Scientific Journal 10(12): 329-337.
- Usharani G, Jayanthi M, Kanchana D, Saranraj P (2014) Effect of bradyrhizobium isolates for the maximization of growth and yield of black gram (Vigna mungo L.). International Journal of Advance Multidisciplinary Research 1(4): 33-39.
- Kim HT (2011) Principles of soil chemistry. (4th edn), pp. 210-220.
- Min J, Lee CW, Gu MB (2003) Gamma radiation dose-rate effects on DNA damage and toxicity in bacterial cells. Radiat Environ Biophys 42(3): 189-192.
- Vilenchik MM, Knudson AG (2006) Radiation dose-rate effects, endogenous DNA damage, and signaling resonance. roc Natl Acad Sci USA 103(47): 17874-17879.
- Chitchanok A, Rattasaritt P, Suthatip S, Suphaporn P, Nattayana P, et al. (2011) Improvement of vitamin B6 production from rhizobium sp. 6-1C1 by random mutation. KKU Res J 16(8): 911-918.
© 2022 Alsaedi SA. This is an open access article distributed under the terms of the Creative Commons Attribution License , which permits unrestricted use, distribution, and build upon your work non-commercially.
 a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in
a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in 







.jpg)






























 Editorial Board Registrations
Editorial Board Registrations Submit your Article
Submit your Article Refer a Friend
Refer a Friend Advertise With Us
Advertise With Us
.jpg)






.jpg)














.bmp)
.jpg)
.png)
.jpg)










.jpg)






.png)

.png)



.png)






