- Submissions

Full Text
Journal of Biotechnology & Bioresearch
Studies on the Effect of Botanicals and Modified Panchagavya Against Chilli Wilt Disease Incited by Fusarium oxysporum
David Kamei1, Archana U Singh2* and Gaichui Gangmei3
1KVK-Senapati, ICAR, India
2Department of Nematology, India
3Department of Agriculture Government of Manipur, India
*Corresponding author: Archana U Singh, Department of Nematology, ICARIARI, New Delhi, India
Submission: January 24, 2022;Published: February 28, 2022

Volume3 Issue4February, 2022
Abstract
Among the different botanical extracts and modified Panchagavya, a natural product tested in-vitro against Fusarium oxysporum an incitant of Chili wilt disease. However, testing was carried out at three different concentrations i.e., at 5%, 10% and 15% and Carbendazim used as check. Garlic extract was found to be the best in inhibiting the growth of the fungus. All the other treatments also showed slow growth rate of fungus as compared to untreated control at all the three concentrations under investigation. It was found that higher concentration had more effect against the radial growth of fungus. Among the three concentrations tested, lowest mean radial growth of fungus was recorded at 15% as 32.99mm followed by 10% as 35.01 mm and maximum radial growth was recorded in 5% as 42.72mm whereas in control it is 90.00mm at 168hrs of incubation period. Among the botanicals and natural product (MPG) treatments, maximum per cent radial growth inhibition was found in garlic (92.91) followed by Darrek, wild sage, nongmangkha, ginger and MPG with percentage growth inhibition of 76.97, 56.80, 56.24, 45.50 and 42.72 respectively over the untreated control.
Introduction
Chilli an important spice crop is known to suffer from various diseases caused by fungi, bacteria, virus, nematodes which reduces yield potential of the crop. Among the fungal diseases wilt disease caused by Fusarium species is a serious one which results in total or partial killing of the standing crops. The symptoms appear as yellowing and wilting of foliage, first starting from the lower leaves and the woody tissue of the stem turns brown and later kill the plant. The fungus initially infect the root system later invades the vascular system of the plant causing discoloration. Sarhan ART et al. [1] worked on the incidence and severity of pepper wilt caused by Fusarium oxysporum f. sp. redolens and Fusarium wilt of chilli caused by Fusarium annum and reported that the disease was characterized by inward and upward rolling of the leaves and wilting of the plant. The disease is soil borne. In Manipur Fusarium wilt disease of Chilli was most prominent and occurs in severe forms. Keeping in view of the residual effect of chemical fungicides on natural environment and human health the present work is taken up in the year 2019-20 based on the use of natural products Modified Panchagavya (MPG) and the indigenous botanical extracts in managing this important Chilli disease.
Materials and Methods
Isolation and identification of pathogen
Chilli plants showing typical wilts symptoms was collected from the farmer’s field and brought in the plant pathology laboratory of KVK-Senapati, Manipur for isolation. Isolation was made from discoloured vascular tissues from collar and root region of an infected plant by cutting into small pieces of 1mm bits. These bits were surface sterilized with 1% sodium hypochloride (NaOCl) solution for two minutes followed by three times serial washing in sterile water. The sterilized bits were then placed aseptically on Potato Dextrose Agar (PDA) medium in sterilized plates and incubated at 27±1 ᴼC for five days. Observations were taken for the development of the fungus and purified by single hyphal tip method.
Preparation of botanical extracts
The aqueous botanical extracts of five different indigenous plants grown in Manipur viz. Garlic (Allium sativum), Ginger (Zingiber officinale), Wild sage (Lantana camara), Darrek (Melia azedirach) and nongmangkha (Plogacanthus thyrsiflorus) was collected and washed with tap water followed by sterile water and shade dry for 24 hours to remove the excess moisture. The plant materials were weighed and crushed with equal quantity of sterile water i.e., 1g per 1ml sterile water (1:1, w/v) with the help of a sterilized motar and pestle. The botanical extracts were filtered in double layer muslin cloth. This formed the standard 100% botanical extracts solution used in the experiments Table 1.
Table 1: Botanicals extracts, MPG, fungicide and their concentration used in the experiment.

Preparation of Modified Panchagavya (MPG)
In Sanskrit Panchagavya means a combination of five products procured from cow (Milk, curd, ghee, dung and urine). It is an ancient ritualistic practice prescribed in Hindu scriptures to administer Panchagavya to human beings under many conditions of ill health, convalescence and as spiritual purifying agent [2].
In the case of our present investigation a Modified Panchagavya (MPG) was prepared following the method of Jahangirdar S et al. [3]. For preparing 1 liter MPG slurry it require the following ingredients cow ghee-20ml, curd-50ml, milk-50ml, cow urine-480ml, cow dung-400g, common salt-20g and backing yeast-10g, all these ingredients were thoroughly mixed and allowed to ferment for 10 days with twice stirring daily. This crude fermented product was diluted 10 times with sterile water and this preparation is now taken to be as 100 per cent standard solution. The diluted preparation was filtered through two layers of muslin cloth and the filtrate was used in the experiment at any desired level of concentration. The pH of the mixture was recorded on zero days and at ten days of the incubation of the mixture.
Effect of different concentration of botanicals and MPG on radial growth of fungus
Effect of different concentration of botanical extracts and MPG on radial growth of Fusarium oxysporum was carried out at three different level of concentration i.e., at 5%, 10% and 15% in the laboratory by following methods of Shivpuri A et al. [4]. The three different concentrations of botanical extracts 5%, 10% and 15% respectively was prepared by pouring 5ml, 10ml and 15ml of each of the standard botanicals extract into each of the 100ml Erlenmeyer conical flasks containing 95ml, 90ml and 85ml sterilized molten Potato Dextrose Agar (PDA) medium aseptically in a laminar flaw. In case of MPG the three different concentrations 5%, 10% and 15% was prepared by passing through a Whatman No. 1 filter paper and from this 5ml, 10ml and 15ml was poured separately into each of the conical flask containing 95ml, 90ml and 85ml PDA respectively to obtain the final concentrations of 5, 10 and 15 per cent. All of these media containing flasks were sterilized in autoclave at 121 ᴼC for 15 minutes. Then 15ml of each concentration of different botanical extracts and MPG was dispensed in sterilized petriplates aseptically and allowed to solidify. Plates containing the medium without extracts served as control. Each plate was then inoculated with a 5mm mycelia disc taken from 5 days old culture of the fungus with the help of cork borer and incubated at 25±1 ᴼC. each treatment was replicated four times. Observation was taken every 24 hours of incubation measuring daily the fungal mycelium growth till it covers the whole plate in control. Per cent inhibition in the growth of fungus for each treatment was calculated by following the method of Vincent JM [5].
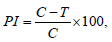
Where PI = per cent inhibition
C = linear growth of fungal in control
T = linear growth of fungus in treatment
The efficacy of all the treatments as well as their best concentration
is determine as per the experimental result provided by
each of the treatment components as compared to control. The
morphological characters of the causal fungus were also studied
during the experiment both in the treatments and control plates.
Results
In-vitro test on effect of different concentration of botanical extracts and MPG on radial growth of Fusarium oxysporum. It is evident from Table 2, that all botanical extracts and MPG at 5% concentration showed slow growth rate of the fungus as compared to control. Among the different treatments, other than Carbendazim, Garlic extracts was found to be best in inhibiting the growth of fungus where the growth occurred only after 120hrs of incubation with 8.5mm. It increased to 14.16mm at 144hrs and to 19.16mm at 168hrs of incubation. Other treatments showed growth of fungus after 48hrs of incubation. However, in Control the fungus growth was observed at 24hrs after incubation (6.5mm) and increased to 90.00mm at 168hrs of incubation. The growth of the fungus in Darrek extract treated plates at following time period of (48, 72, 96, 120, 144 and 168) hours, were (6.5, 14.16, 22.00, 31.33, 35.80, and 39.50) mm respectively and in case of Wild sage (7.33, 14.33, 22.50, 31.66, 36.50, 39.83) mm Nongmangkha (8.16, 16.66, 24.36, 32.50, 37.50 and 41.50) mm, Ginger (8.83, 19.15, 28.66,39.50,47.33 and 53.00) mm and MPG (10.50, 23.16, 31.50, 43.63, 52.50 and 58.83) mm respectively. There was no fungal growth in Carbendazim treated plates. Among the different treatments, Garlic extracts was found to be best (5.97mm) followed by Darrek (21.32mm), Wild sage (21.73mm), Nongmangkha (22.95mm), Ginger (28.11mm) and MPG (31.44mm).
The experimental data presented in Table 3 also indicated that different plant extracts and MPG at 10% concentration showed slow growth rate of test fungus as compared to control. There was no growth in garlic extracts and Carbendazim treated plates. In all other treatments growth of the fungus appeared after 48 hours of incubation as compared to 24 hours in control. In Darrek treated plates the growth of test fungus at (24, 48, 72, 96, 120, 144 and 168) hours were (5.50, 7.83, 10.66, 13.16, 15.83 and 17.00) mm respectively followed by Wild sage (6.50, 13.83, 21.16, 30.80, 34.66, and 38.16) mm, nongmakha (6.66, 15.16, 21.83, 31.16, 34.83, 38.66) mm and MPG (8.16, 18.50, 27.66, 35.16, 43.50 and 49.66) mm respectively. In this 10% concentration Garlic extract was found best in inhibiting the fungal growth followed by darrek (9.99), wild sad sage (20.73mm), nongmangkha (21.18mm), ginger (25.25) and MPG (26.09) as compared to 50.44mm in control plate.
The experimental data presented in table 4 indicated that Garlic extract at 15% concentration and Carbendazim showed complete inhibition of growth of Fusarium oxysporum. Darrek at 15% concentration showed growth only after 72 hours of incubation with 3.50mm which increased to 4.60mm, 5.20mm and 5.66mm at 144 hours of inoculation with no further growth thereafter. All other treatments at 15% concentration also showed delay in growth as compared to control. At (48, 72, 96, 120, 144 and 168) hours, the growth of fungus in Wild sage treated plates were (5.66, 14.00, 22.16, 31.00, 34.16, and 38.00) mm respectively, in nongmangkha (6.00, 13.16, 22.60, 31.16, 34.80 and 38.66) mm, in Ginger (7.16, 16.5, 25.16, 32.50, 40.33 and 46.00) mm and MPG (7.33, 16.66, 25.33, 33.66, 40.83 and 46.16) mm respectively.
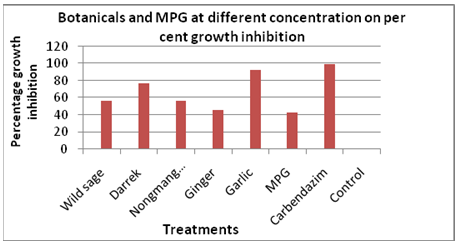
It is also evident from Tables 2, 3 & 4 that wild sage and nongmangkha extracts were found non significance among themselves but significance over control in their effectiveness against the growth of test fungus. (Figure 1). The data presented in the Table 5 is the experimental results of botanicals and MPG at three concentrations (5%, 10% and 15%) on radial growth of fungal mycelium and the percentage inhibition of the test fungus (Fusarium oxysporum) over the control. Data revealed that Garlic extract was found most effective in inhibiting the fungal growth at all three concentrations with mean mycelial growth of 6.38mm with per cent growth inhibition of 92.91 followed by Darrek (20.72mm), Wild sage (33.88mm), nongmangkha (39.38mm), Ginger (49.05mm) and MPG (51.55mm) as compared to control (90.00mm) with their respective per cent growth inhibition of 76.97, 56.80, 56.24, and 42.72 over control.
Table 2: Effect of different botanicals extracts at 5% concentration on growth of F. oxysporum at different period of incubation.

Table 3: Effect of different botanicals extracts at 10% concentration on growth of F. oxysporum at different period of incubation.

Table 4: Effect of different botanicals extracts at 15% concentration on growth of F. oxysporum at different period of incubation.

Table 5: Efficacy of different concentration of botanical extracts and modified panchagavya (MPG) on radial growth of F. oxysporum.

Figure 1: Botanicals and Modified Panchagavya (MPG) and per cent growth inhibition of Fusarium oxysporum at different concentration.
Figures in parentheses are square root transformed values (√x+0.5).
Discussion
The pathogen of the present Chilli wilt disease under investigation was confirmed as Fusarium oxysporum after isolation and purification in the laboratory of plant pathology KVK-Senapati, Manipur. Sharhan and Sharif (1988) also reported that Fusarium wilt of Capsicum was caused by F. oxysporum f. sp. redolens whereas Lukacs J et al. [6] reported F. oxysporum and F. Solani as the causal organism.
It is evident from Tables 2-4 that all botanical extracts and MPG showed slow growth rate of the fungus as compared to control of all concentration tested. Among different botanicals and MPG treatments Garlic was found to be most effective against the growth of fungus where growth occurred only after 120hrs of incubation with 8.50mm and increased to 14.16mm at 144hrs and 19.16mm at 168hrs of incubation at 5% concentration in Table 2. However, at 10% and 15% concentration it completely inhibits the growth of the test fungus. The finding are in support of Tripathi AK et al. [7] who reported that the extracts of Allium sativum completely inhibit the radial growth and sporulation of Alternaria lini under in-vitro conditions by poisoned food technique. Tabin T [8] observed that garlic and ginger extracts were highly effective in inhibiting the spore germination of Erysiphe polygoni causing powdery mildew of pea. Gohil VP et al. [9] observed that aqueous extract of Allium sativum and Sopindus trifoliate inhibit mycelium growth of Fusarium moniliforme.
Studies on effect of different concentration of botanical extracts and MPG on radial growth of F. oxysporum showed that all three concentrations tested reduces radial growth of the fungus (Table 5). The efficacy of all the plant extracts and MPG tested increased with increase in concentration. It is thus found that among the botanicals Garlic was found most effective against the growth of fungus at all the three concentration viz. 5%, 10% and 15% whereas mean mycelial growth was 6.38mm followed by Darrek (20.72mm), wild sage (33.88mm), nongmangkha (39.38mm), ginger (49.05mm) and MPG (51.55mm) as compared to control (90.00mm). Singh UP et al. [10] also observed that among the three concentrations of ginger extracts (1000, 1500 and 2000) ppm against powdery mildew disease of pea, the highest dose 2000ppm gave the best result inhibiting the disease (30.20%). Bansal RK et al. [11] also found that among seven leaf extracts with different concentrations tested found that Azadirachta indica was highly toxic to F. oxysporum with complete inhibition of mycelial growth and spore germination at highest concentrations of 100% followed by Ocimum bacillium and Lantana camara. Singh S et al. [12] also reported that leaf extract of neem at 100% concentration completely checked the spore germination of F. oxysporum f. sp. ceceri while 100% of garlic bulb extracts could only germinate upto 1.7%.
The highest mean growth inhibition percentage over control was observed in Garlic extract (92.91%) followed by Darrek (76.97%), wild sage (56.80%), nongmangkha (56.24%), ginger (45.50%) and MPG (42.72%). Sarma R et al. [13] tested aqueous extracts of 15 weeds at 5%, 10% and 20% concentration against Rhizoctonia solani causing sheath blight of rice and observed that the highest concentration (20%) was found superior to others.
Conclusion
This study shows that different concentration of botanical extracts and Modified Panchagavya reduces the radial growth of the Fungus and helpful in reducing the wilt disease caused by Fusarium oxysporum in Chilli. Thus, the productivity of Chilli crop can be increased when Wilt problem is not there in crop.
References
- Sarhan ART, Sharif FM (1986) Integrated control of Fusarium wilts of pepper. Acta Phytopathologica et Entomologica Hungarica 21(12): 123-126.
- Reddy HR, Padmodaya B (1996) Test to control the Fusarium wilt disease of tomato with various formulations show that Panchagavya (MPG-3) is the most effective. Down to Earth, New Delhi, India.
- Jahangirdar S, Siddaramaiah AL, Ramaswamy GR (2001) Influence of biocontrol agents and MPG-3 on Fusarium oxysporum Sp. cubense incitant of Panama disease of banana. Pl Dis Res 16(1): 68-72.
- Shivpuri A, Gupta RBL (2001) Evaluation of different fungicides and plant extracts against Sclerotinia sclerotiorum causing stem rot of mustard. Indian Phytopath 44(1): 55-59.
- Vincent JM (1927) Distortion of fungal hyphae in presence of certain inhibitors. Nature 159(4051): 850.
- Lukacs J, Czarka J (1988) Fusarium wilts of capsicum. Zoldsegtermesgtesi Kutato Interzet Bulletin 22: 95-99.
- Tripathi AK, Verma KP, Agrawal KC, Shukla RSS (2002) Effect of plant extracts on mycelia growth, sporulation and spore germination of Alternaria alternata under in-vitro J Mycol Pl Pathol 32(2): 268-269.
- Tabin T (2005) Influence of plant extracts on powdery mildew (Erysiphe polygoni DC) of Pea cv. Makhyatmubee in Manipur (Agri) Thesis, Central Agricultural University, Imphal, India, pp. 22-24.
- Gohil VP, Vala DG (1996) Effect of extracts of some medicinal plants on the growth of Fusarium moliniforme. Indian J Mycol Pl Pathol 26(1): 110-111.
- Singh UP, Srivastava BP, Singh KP, Mishra GD (1991) Control of powdery mildew of pea by ginger extract. Indian Phytopath 44(1):55-59.
- Bansal RK, Gupta RK (2000) Evaluation of plant extracts against Fusarium oxysporum wilt pathogen of fenugreek. Indian Phytopath 53(1): 107-108.
- Singh S, Chand H (2004) Effects of extracts of some medicinal plants on spore germination of chickpea wilt pathogen. Indian J Pl Protec 32(1): 162-163.
- Sarma R, Phookan AK, Bhagawati KN (1999) Efficacy of some plant extracts in the management of sheath blight disease of rice. J Mycol Pl Pathol 29(3): 336-339.
© 2022 Archana U Singh. This is an open access article distributed under the terms of the Creative Commons Attribution License , which permits unrestricted use, distribution, and build upon your work non-commercially.
 a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in
a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in 







.jpg)






























 Editorial Board Registrations
Editorial Board Registrations Submit your Article
Submit your Article Refer a Friend
Refer a Friend Advertise With Us
Advertise With Us
.jpg)






.jpg)














.bmp)
.jpg)
.png)
.jpg)










.jpg)






.png)

.png)



.png)






