- Submissions

Full Text
Journal of Biotechnology & Bioresearch
Projected Disposal-Geared Design of Arsenic- Removing Media for Batch Reactor, Evaluation of Engineering Design, and Review of Disposal Options for Foul Arsenic-Laden Media
Lucina Márcia Kuusisto* and Malayshia Pouncil
Texas A&M University-Commerce, USA
*Corresponding author: Texas A&M University-Commerce, USA
Submission: January 28, 2021;Published: February 23, 2021

Volume2 Issue5February, 2021
Abstract
Arsenic is one of the eight metals regulated under RCRA (Resource Conservation and Recovery Act).
Arsenic can cause many health problems, including skin cancer, lung cancer, and bladder cancer. It is also
known to be a cause of diabetes, pulmonary and cardiovascular diseases. The US EPA stipulates the limit
for arsenic levels in the drinking water to 0.01mg/l or 10 parts per billion (ppb).
The main goals of this research involved the following 3 main objectives:
A. The first goal involved the engineering design evaluation of the water filter media.
B. The second goal encompassed analyzing the role of lingocellulosic fiber in the capability
increase of particle adsorption and abstraction in an optimized water treatment media, purposed for
arsenic removal.
C. The third objective comprised of studying the ultimate environment-benign disposal of the
arsenic-laden foul media.
In view of the intended eventual disposal method for this media, other sought-after properties of its components included: ability to foment biochemical reactions, porosity, and biodegradability. The projected method for arsenic abatement and eventual disposal was through phytoremediation. The media contained lignocellulosic fiber (dehydrated Zea mays or corn husk) and coconut shell PAC (Powdered Activated Carbon), as part of its makeup. The efficacy of this arsenic-removing media was measured. Different disposal alternatives for arsenic-laden media were defined. The filter media components were measured and assembled by using the following ratios of PAC and corn husk: 75:25 and 50:50 ratios. A constant mass of an anthracite layer was used. Initially, a constant mass of Fe2O3 was placed on the top layer of the filter media. The estimated grade of our newly designed water filter, deduced after juxtaposing its features to the regulatory design criteria, was, approximately, equal to 75%. As expected, the total mass of the spent media decreased with the lower ratio of lingocellulosic fiber. Both ratios of PAC and corn husk removed arsenic in comparable manner, but at different rates. The lowest turbidity was observed when the Fe2O3 was removed from the media.
Introduction
Arsenic can enter the water, naturally, from deposits in the earth’s crust, and is naturally found in soils and minerals. It is possible to enter the air, water, and land from dust blown by wind, and it can react with water through runoffs and leaching. It contaminates the environment form anthropogenic practices, including mechanical and farming contamination. Chemical reactions, between arsenic compounds and hydrogen in water or acid, produce arsine, a very dangerous lethal gas. Arsine is lethal if the person undergoes a 1-hour exposure at the level of 0.50ppm, or, a worse scenario may happen, during a shorter period of time, within a 10-minute exposure, at a higher arsine concentration of 0.91ppm [1]. According to the Agency for Toxic Substances and Diseases Registry (ATSDR) [2] many workplaces, using inorganic arsenic, have to limit the airborne arsenic exposure of their employees, working an 8-hour shift, to 10µg/m3. This PEL (Permissible Exposure Limit) of 10µg/m3 has been established by the Occupational Safety and Health Administration (OSHA). According to the World Health Organization [3] arsenic (As) contamination in water may cause the following illnesses: Skin cancer, skin lesion, and can lead to chronic arsenic poisoning (arsenicosis). Consuming arsenic over a long period of time can result in long term health effects including diseases found in blood vessels, diabetes, bladder, kidney, and lung cancer.
Treatment Alternatives to Remove Arsenic from Water
In water, arsenic can bond, chemically, and form organic and inorganic compounds. The inorganic arsenic compounds are, generally, more toxic. There are two states of the inorganic compounds: Arsenite (iii) and Arsenate (v). According to [4] during the treatment studies of arsenite and arsenate with ferrihydrite compounds, the outcome of the arsenite adsorption processes was a net release of hydroxide ions (OH-) at a higher pH level of 9.2, and a net release of Hydronium ions (H+) when the pH was as low as 4.6. Conversely, during similar treatment conditions, the arsenate adsorption processes ended up on a net release of hydroxide ions (OH-) at both pH levels of 4.6 and 9.2. After their experiments, and maintaining the pH range between 4 and 10, [4] concluded that the adsorption processes on Fe ions were lower under the following conditions: (i) For the arsenite species, when the pH was low. (ii) For the arsenate species, when the pH was high.
Previous studies have shown that arsenic can be removed from water via co-precipitation with other elements, including iron and aluminum. In addition, adsorption of arsenic onto porous surfaces has shown to have successful results on the arsenic removal process (Tamaki and Frakenberger, as referred to by [5]). Chemical reactions of arsenic in water may occur in different oxidation states. Arsenic may exist under high pH and oxidizing conditions. High pH leads to desorption of absorbed Arsenic. When the pH is equal to 9.2, arsenite exists as a 50-50 mixture of H3AsO3 and H2AsO31- [6]. Moreover, arsenic exists in a chemically reduced form in aquifer environments (low oxygen waters). The chemical oxidative form of the arsenic species depends on the reduction/oxidation reactions (redox potential), and pH. Arsenic can also be combined with other elements. Some of these elements are also pollutants, originating from wood preservatives, and others from insecticides, used in cotton farms and other agricultural crops [7] Figure 1 shows the geographic areas where arsenic levels are high in the United States.
Figure 1:USGS map of locations with high arsenic levels Credit: U.S. Geological Survey. Department of the Interior/USGS.
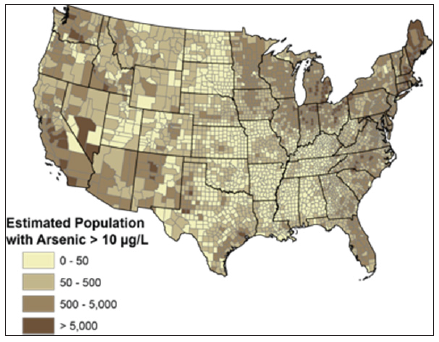
Many areas of the world are assailed with arsenic contamination. There has been historical arsenic contamination problems in the City of Commerce, Texas. The arsenic contamination originated, mostly, from the defunct Hi-Yield chemical plant, a manufacturing company that produced arsenic-based herbicides, insecticides, and pesticides. The vicinity of the former Hi-Yield chemical plant was declared a superfund site by the US EPA, due the high levels of arsenic contamination in that area. In the 1990’s the measured arsenic levels in the soil were reported to be as high as 300ppm, whereas the mean background measurements from that region’s uncontaminated soil had been 3.2ppm (Fife, 1995, TNRCC, 1992, as referred to by [5]. Then, by 1996, the US EPA empowered the excavation of the soil for off-site disposal, and placed the remaining contaminated soil in a ditch, and then, surrounded the contaminated soil by slurry, and, then, the US EPA finalized the remediation process by capping the soil mound with clean soil and vegetation [8].
Currently, the most common types of treatment to remove arsenic from water include adsorptive media, reverse osmosis, distillation, and anion exchange. Additional technologies include electrocoagulation, and anaerobic removal with iron sulfides [9]. Previous studies of arsenic removal from water included the following types of treatment processes: use of iron oxide-coated sand, ferric chloride, followed by coagulation. The effectiveness of each treatment varies, depending on the chemistry of the water being treated. According to [10], iron-based adsorbents have been, successfully, used as arsenic removing agents from water. [11], studied the use of suspensions of hydrous ferric oxide to achieve the chemical reduction of iron (iii) and arsenic (v), simultaneously, with the aid of microorganisms. In addition, in order to accomplish the oxidation reaction, it is crucial to have a chemical oxidant to foment the oxidation reaction [12,13] studied the chemical removal process of arsenic from water by adding strong oxidants, such as NaOCl or KMnO4, and, in some cases, FeCl3, before removing the arsenic from the water. Furthermore, coagulation with the addition of ferric or aluminum salts improve arsenic removal, and the type of arsenic oxidation state also affects the arsenic removal rates by enhanced coagulation, i.e., arsenic (v) is, usually, removed by coagulation in a more effective manner than As (iii) [7,13]. Moreover, other treatment techniques include the removal of arsenic through adsorption and precipitation, followed by filtration. Initially, As (iii) is oxidized. Then, As (v) is adsorbed onto the FeO precipitates, thus increasing the size of the particles. Finally, the combined precipitates are filtered from the solution [12].
Implications
This research is the first, to the authors’ knowledge, involving the specific design of an intended disposal-geared design of bio-physicochemical reaction-enabling filtering media for arsenic removal from water. This research studied the role of lingocellulosic fiber in the increase of particle adsorption and abstraction capability in this water treatment media. In addition, this research examined different disposal alternatives for arsenic-laden foul filter media, describing specific projections for phytoremediation. Discussions about the viability of further treatment, reutilization, and disposal of the arsenic-laden foul media are included. Furthermore, laboratorial tests were performed to determine whether different ratios of Powdered Activated Carbon (PAC) and lingocellulosic fiber (dehydrated Zea mays or corn husk) affected the media efficacy in removing arsenic from water. Arsenic is one of the eight metals regulated under RCRA (Resource Conservation and Recovery Act). High levels of arsenic can cause many health problems. The information contained in this study will be useful to decision-making professionals regarding (i) the engineering design of cost-effective, organic-bond enabling filtering media for arsenic removal from water; (ii) the selection of sustainable “green” components of water filter media; (iii) the environment-oriented disposal options of arsenic-laden wastes.
Objectives
Different types of water treatment technologies for the removal
of arsenic from water have been broadly researched. In addition,
the toxicity, transformations, and transport of arsenic-containing
waste have also been thoroughly studied [14].
The main goals of this research involved the following 3 main
objectives:
I. The first goal involved the engineering design and
evaluation of the water filter media, following the standards of the
engineering design criteria for treatment equipment [15].
According to the US EPA, the engineering design criteria for
treatment equipment includes the following:
a. Cost;
b. Complexity;
c. Robustness;
d. Availability of Equipment and Process Technology;
e. Pretreatment;
f. Residuals;
g. Through-put Potential;
h. Scale-Proven;
i. Ease of Permitting and Public Acceptance.
The estimated grade of our newly designed water filter, deduced after juxtaposing its features to the regulatory design criteria, was, approximately, equal to 75%.
II. The second goal encompassed analyzing the role of lingocellulosic fiber (dehydrated Zea mays or corn husk) in the capability to increase of particle adsorption and abstraction in an optimized water treatment media, purposed for arsenic removal. In addition, this research discusses the design, setting up, and testing the effectiveness of this physicochemical reaction-enabling filter to remove arsenic from water. These design plans targeted the components of the filter media to enable physical and chemical reactions to accomplish such goal. The newly designed filter media included coconut shell PAC (Powdered Activated Carbon), dehydrated corn husk, and Fe2O3 (iron (iii) oxide) as part of the filter makeup. The inherent main difference of this particular prototype is its arsenic-removing media, which had been specifically designed to be sustainable, cost-effective, and biodegradable for further disposal.
III. The third objective comprised of studying the ultimate
environment-benign disposal of the arsenic-laden foul media.
In view of the intended eventual disposal method for this media,
other sought-after properties of its components included: ability
to foment biochemical reactions, porosity, and biodegradability.
The projected method for arsenic abatement and eventual disposal
was through phytoremediation. Furthermore, this study includes
an in-depth discussion of treatment and disposal of foul arseniccontaminated
waste. In addition, other valuable information,
derived from this research includes the results from testing this
newly designed water filter media, projected to enable biochemical
bonds of arsenic, during its foul-media disposal, thus stabilizing the
arsenic in non-edible floral plantations. Further research is needed
about the microorganism activities of organic arsenic in the soil.
Arsenic is one of the eight metals regulated under RCRA
(Resource Conservation and Recovery Act). High levels of arsenic
can cause many health problems.
The results of this research will be useful for environment
benign decisions concerning the following:
a. engineering design of cost-effective, organic-bond enabling
filtering media for arsenic removal from water;
b. selection of sustainable “green” components for water
treatment; and
c. environment-oriented disposal alternatives of arsenic-laden
wastes.
Phase 1: Engineering this Physicochemical Reactionenabling Water Filter Media
Usually, during the physicochemical treatment for arsenic removal from liquid waste, the current practices use two or more steps to accomplish such feat. The physicochemical treatment methods for arsenic removal from liquid waste include chemical precipitation, chemical oxidation, and coagulation with CaO (calcium oxide). These processes have many disadvantages, including high cost and high energy consumption [16]. In order to decrease the cost of operations, and high energy consumption, the team working on this research devised a one-enclosure type of physicochemical reaction-enabling “handy” water filter for arsenic removal.
Formulation of filter media in treatment equipment
During the conceptual phase of any engineering design, the
available information is, usually, qualitative, and, not exact. However such conceptual incomplete information poses to be insufficient for
the future replication of the item being conceptualized. Therefore,
in order to replicate the newly designed item, the engineer must
generate quantitative and accurate information, and prepare
an Engineering Design Specification report. Although some
conceptualized items will, most likely, suffer modifications, during
the entire idea-to-product process, the Engineering Specifications
contain crucial information for the subsequent phases, e.g.,
prototyping, testing, and manufacturing [17].
The specification of this filter media engineering design has
been organized in the following categories:
In-use purposes
1. The primary purpose of this water filter media is to remove
arsenic from purified drinking water.
2. Additional special purpose of this water filter media includes
to ensure the ultimate environment-benign disposal of the
arsenic-laden foul media.
3. Unintended purpose: This water filter media has not been
designed to treat wastewater.
Functional design parameters
1. Capacity: Filtered water volume of 2 Liters;
2. Input/output conditions:
a. Temperature: 68-72 °F (20-22 °C);
b. Required water flow: Gravity flow conditions;
c. Power: No outside energy source is required for
operations.
3. Efficiency: The removal efficiency was calculated using the
following equation: (initial concentration-final (filtered water)
concentration)/initial concentration. The initial removal
efficiency was 15%.
4. Physical attributes, Encasing of filter media: This water filter
media was designed to serve as a complimentary filter, to be
inserted in the filter casing of a portable household water filter
pitcher.
Rationale for selecting filter media components
The rationale for selecting the media constituents included deciding to utilize the following properties of each constituent:
Fe2O3 (iron (iii) oxide), to aid in the redox chemical reactions with arsenic: Many scientists have, successfully, used the iron ion to remove arsenic from water. Customary practices to accomplish such a feat include using both ferric chloride (FeCl3) and ferric sulfate (Fe2(SO4)3). [18] have studied that by infusing iron in activated carbon particles, the arsenic removal rates increased. Furthermore, [4] concluded that the adsorption processes of arsenic onto Fe ions varied with the pH.
PAC (Powdered activated carbon): manufactured from coconut shells, is a key component in advanced water treatment facilities. The decision to include PAC in this filter media combination derived, mainly, from the fact that PAC includes low density and high porosity among its positive properties. Furthermore, in this study, the purpose for adding PAC to the media composition included:
a. Increase the adsorption capacity of the filter media.
b. PAC is organic, and, after being used as an adsorbent, it can be re-used, later, to immobilize the arsenic, and function as compost, for non-edible ornamental flowers and shrubs. The environment-friendly rationale for the final fate of this compost is the following: In view of the fact that the arsenic present in the soil may infiltrate in plant roots and cells [19], treating and using the arsenic-contaminated compost for non-edible ornamental flowers, poses as a safe and viable way of immobilizing the arsenic.
The reasoning for including the dehydrated corn husk was also two-fold:
a. One of the main positive aspects of this material is the fact
that its pores are so closely tight and small, that it may act as a quasimicrometrical
layer. Current technology recommends the use of
non-plastic materials in the manufacturing of environment-friendly
products. Additional advantages of using lignocellulosic fiber,
include the facts that cellulose-based materials are sustainable, and
cost-effective [20].
b. Dehydrated corn husk is an organic biodegradable
material, and, after being decomposed by microorganisms, it may
also act as compost, after being used as part of the arsenic-filtrating
media. Similarly to PAC, the dehydrated corn husk-containing
compost may be treated, and used for non-edible ornamental
flowers and shrubs.
The selection of anthracite derived from its basic inherent properties:
a. Enhanced sorption opportunities due to its increased
specific surface area, its angled shapes, and its high percentage of
void space.
b. The strength of this material availed sufficient physical
structure to support the weight of the other media components.
Engineering design criteria for filter media in treatment equipment
This phase encompassed the designing, setting up, and testing
this water filter, containing physicochemical reaction-enabling
media for arsenic removal.
1. Main filter efficacy determinants have been assessed by
researching the following information:
Comparison of this researched optimized equipment design
versus the Engineering Design Criteria for Treatment Equipment
[15];
Table 1: Comparison of this researched equipment design versus the regulatory Engineering Design Criteria for Treatment Equipment.
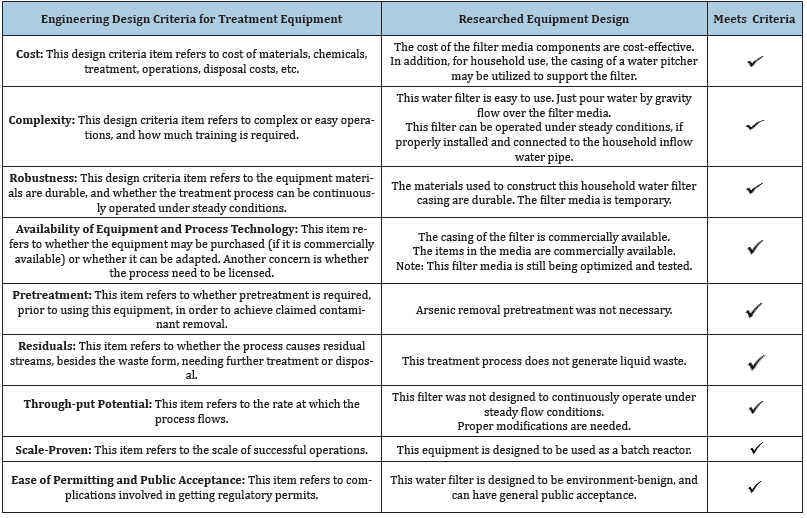
2. Results from testing the arsenic removal efficacy. Table 1 displays the comparison of this researched equipment design versus the regulatory Engineering Design Criteria for Treatment Equipment. According to the [21], the Engineering Design Criteria for treatment equipment includes the following:
Phase 2: Laboratory Experiment
Materials and methods
Arsenic-contaminated stock solutions were prepared in the Lab. The glass water bottles had been, previously, thoroughly cleansed with 0.1mol/L nitric acid, and then, rinsed with deionized water. Then, the sample-containing glass bottles were labeled, and placed in the Environmental Science Lab refrigerator, until future analyses.
Preparation and setup of filter media in treatment equipment: The components of the filter media were measured and assembled by using the following ratios of PAC and dehydrated corn husk: 75:25 and 50:50 ratios. A constant mass of dehydrated corn husk and anthracite layers were used, during each filtering and testing period. The dehydrated corn husk was maintained above the anthracite, and both of these types of media were kept at an approximate total depth of 0.5 inch (1.27cm). The mass of filter media components were weighed at an analytical balance. In order to facilitate the physical support of the filtering media in the filter casing, an inert-material paper coffee filter was inserted underneath the media being tested. Then, the arsenic-contaminated water was conveyed through each composition of the filtering media. Initial and final arsenic levels were measured, before and after each filtrating run.
Figure 2: Water pitcher, used to house the filter media.

A transparent acrylic water filtering pitcher, commercially
known as Brita Ultra Max TM, was used to physically house the
filter media. This novel filter media was inserted in the filter casing
of water filter pitcher, replacing the original enclosed filter. Figure
2 displays the water pitcher used to house the filter media for
these experiments. During each filtration run, the pitcher casing
was filled with the filter media. The overall dimensions of the
laboratory-scale water pitcher were: 14.37 in x 5.67 in x 10.47
inches or, approximately, 36.50cm x 14.35cm x 26.59cm.
The temperature was maintained at 73 °F (22.8 °C), during the
experimental phase. The pH of the experiment was maintained
at 6, and adjusted, if necessary, by using 1M NaOH and 1M HNO3
[22]. The commercial brand of the instrument used to measure the
pH was the Benchtop pH/MV Meter, Speer Scientific. In addition,
1000mg/L of arsenate stock solution, prepared in the following
manner: deionized water plus Na2HAsO4·7H2O. During the
experimental phase, the arsenic content was measured by using the
US EPA-approved test kit, commercially known as Quick™ Arsenic
Test Kit (ITS 481303 Arsenic Quick II).
Simulation of arsenic-contaminated water filtration: The initial arsenic concentration levels were set in the following range: from 5 to 25ppb (μg/L).
A previously calculated number of water samples, each containing specific measures of initial arsenic concentrations, each measuring up to 200mL, were conveyed into each different composition of the filter media, inside the water pitcher. These routines were repeated for 3 observation-days. The initial gravity flow rate for the 75:25 PAC and husk ratio was, approximately, 0.20L/ min. After each filtration run, the residual arsenic concentration was measured. Three replicates of arsenic concentrations were measured before and after filtration for each media composition. In order to account for experimental errors, the mean value, among the triplicates results, was selected.
Arsenic removal efficiency calculations
The removal efficiency was calculated using the following
equation:
(initial concentration - final (filtered water) concentration)/
initial concentration.
Results and discussion
During the 4-day time period of laboratorial tests, the removal
efficiency was 100%.
Both ratios of media (75:25 and 50:50 ratios of PAC and
dehydrated corn husk) yielded similar removal efficiencies
throughout the tests.
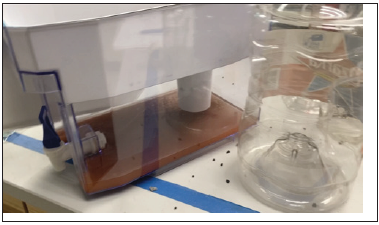
Engineering Design Issues: (a) Layer of Fe2O3 in the Same Chamber; and (b) Inadequate Pre-treatment: One of the media components, iron (iii) oxide, did not contribute to successful filtration results. The powder turned to a consistency of clay once water ran through the filter. The aesthetic quality of the filtered water decreased with the presence of Fe2O3. This dark water color caused color reading difficulties. The only arsenic measurements taken were those from the unfiltered water. The final values of arsenic could not be observed. Figure 3 displays the dark color of the filtered water, due to the presence of Fe2O3 in the media.
Figure 3: Dark color of filtered water, due to presence of iron (iii) oxide mixed with the other filter media components.
According to former studies, the chemical reactions enabled
by Fe2O3, requires the presence of an oxidant, and lower pH values.
However, lowering the pH and adding an oxidant to the unfiltered
water, would have changed the ultimate purpose for this study.
Therefore, this research team decided to maintain the pH at 6, and
forgo the oxidant. In view of these impasses of not varying the pH,
and not adding an oxidant, and in view of the fact that the presence
of Fe2O3 contributed to a decrease of the aesthetical properties of
the filtered water, that component (Fe2O3) was completely removed
from the filter media.
The results indicating the lowest quantity of arsenic were
observed from the samples filtered by the media, excluding iron
(iii) oxide. Furthermore, the idea of using an increased mass of
dehydrated corn husk resulted in a low filtration rate. Although the
arsenic had been removed, it was observed that the rapid clogging
of the filter occurred when the corn husk ratio was higher. Figure 4
displays the corn husk being re-arranged. As previously discussed,
according to observations, the higher percent mass of corn husk
in the media contributed to lower filtration rates. Figure 5 shows
that an increase of filter porosity (decrease of corn husk mass)
resulted in an increase of filtration rate. Later, during the remaining
period of the experimental phase of the 1-step type of treatment,
the filtered water had better (clearer) aesthetical quality, and there
was a positive trend in the removal of arsenic. At that time, as water
was conveyed and passed through the new filter, a sizzling noise
was heard from the activated carbon location.
The inference statement from the lab experiment is the following:
After removing Fe2O3 from the filter media, the combined properties
of the remaining components imparted sufficient capacity for them to function as effective sorption agents, and no arsenic level was
found in the filtered water. Figure 6 displays the potential arsenic
removal efficiency for low contaminant concentration of the newly
assembled filtering media, which excluded the iron (iii) oxide from
the media. The positive trend in arsenic removal was achieved by
the sorption, and other physical filtering processes enabled by the
PAC, the dehydrated corn husk, and the anthracite. The filtering
efficacy of sorption surfaces enabled the adhesion properties at the
PAC surface areas to adsorb the contaminants. Furthermore, the
additional angled surfaces of the anthracite, distributed throughout
the rocks, created further opportunities for contaminant
entrapment, as the water flowed through the media. The presence
of dehydrated corn husk contributed to a low filtration rates, and
its nearly micrometrical-sized pores served as optimal structures
to further capture the contaminants. The newly assembled filter
media caused the arsenic levels to decrease to zero from as high
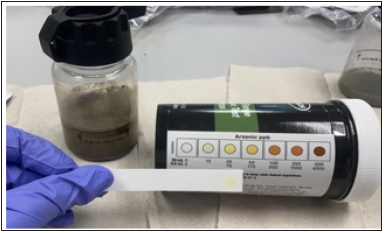
as 25ppb. Figure 7 displays the initial arsenic level was 25ppb for
a 50mL water sample, measured in accordance with the dual range
arsenic test strips.
Figure 4: The higher percent of corn husk in the media resulted in a decrease of filtration.

Figure 5: Increase of filter porosity (decrease of corn husk) resulted in an increase of filtration rate, excluding Fe2O3.
Figure 6: Potential removal efficiency for the filter media excluding Fe2O3.
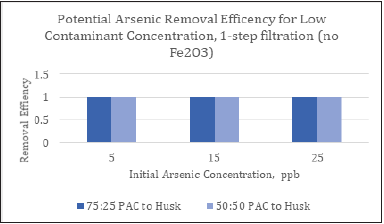
Figure 7: Potential removal efficiency for the filter media excluding Fe2O3.
The mean concentration of arsenic in ppb was calculated for each sample. The difference between arsenic levels, before and after filtration, was as high as 25ppb. The remaining components of the filter media (PAC, dehydrated corn husk, and anthracite) worked as an effective arsenic removal combination for a one-equipment type of filter, treating low initial levels of arsenic. All the average results were below the US EPA mean level of 10ppb for arsenic in water.
Phase 3: Environment-benign Disposal Options for Arsenic-laden Foul Media
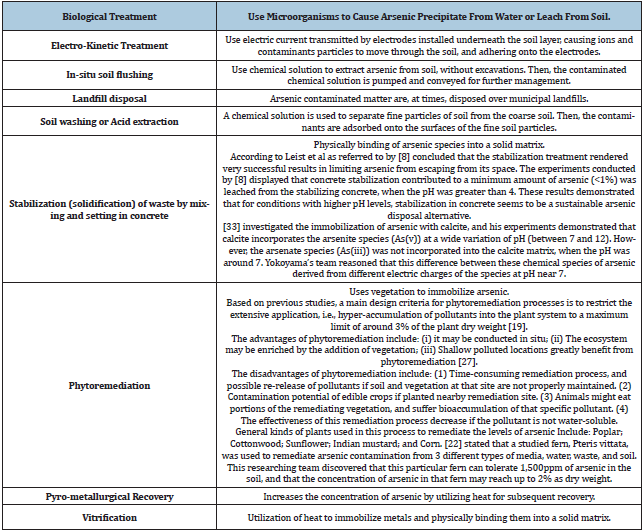
Table 2 displays the main alternatives for treating and disposing arsenic-laden waste, as published by the US EPA in 2002 [21].
Table 2: Main alternatives for treating and disposing arsenic-laden waste [27].
Recommendation for Viable Treatment and Disposal of this Researched Filter Media, after it becomes Foul and Arsenic-laden
The foul arsenic-laden media, used in this study, may be skimmed from above the anthracite layer, and, then, be hauled out, and act as compost. This compost may be used for non-edible ornamental flowers and shrubs. In view of the fact that the arsenic present in the soil may infiltrate in plant roots and cells [19], using arsenic-contaminated compost to non-edible ornamental flowers, poses as a safe and viable way of immobilizing the arsenic.
Future Studies
Future research should be conducted to test iron (iii) oxide
capabilities as an arsenic removing agent in a one-equipment type
of drinking water filter. These physicochemical reactions should
be conducted in a separate compartment, inside the casing, before filtration occurs. It has been established that an oxidant should
be added, and a lower controlled pH range should be part of the
variables. For arsenic removal purposes, during future engineering
designs of the geometric shape and dimensions of a one-equipment
[“household”] water filter, it is crucial to optimize the physical
design of the filter casing by including a separate enclosed cylinder
to house the Fe2O3.
In order to empower all of these physicochemical reactions in
a single container, the following processes should be enabled and
optimized:
a. Foment oxidation reactions;
b. Permit longer contact time for the precipitates to settle;
c. Enable adhesion of precipitates into adsorbents surfaces;
d. Attain maximum arsenic removal capacities.
Conclusion
As previously discussed, another alternative for treating and disposing arsenic-laden waste includes ion exchange. During his studies, [23] concluded the following: After treating the liquid waste from a smelter processing industry, the foul metal-laden sludge may be hauled out, placed in a container, and diluted for further treatment. Then, copper and cobalt may be separated from the arsenic in the smelter processing solution by resorting to the chemical reactions of some specific ion-exchange resins [23], reported to have removed and separated copper and cobalt from arsenic in an acid pressure leach solution, by using the following two resins: Cu-WRAM and WP-2. During the design phase of this filter, this physicochemical reaction-enabling filter should have had an enclosed device to minimize the gravity flow, thus permitting more time for the initial oxidation step. According to [24] the ferrihydride process is a main component used to successfully remove arsenic from drinking water. However, the ferrihydride process requires an initial oxidation step for increased arsenic removal. Therefore, for future designs of small [or portable] drinking water treatment equipment, one should include this oxidation initial step, perhaps, in a separate inner cylinder, but inside the same casing [25-30]. In addition, further research is recommended on different treatment and disposal alternatives of foul arsenic-laden media, and/or regeneration solutions from ion-exchange resins [31,32].
Data Availability
Some or all data, models, or code that support the findings of this study are available from the corresponding author upon reasonable request.
Acknowledgment
The authors gratefully acknowledge Dr. Deidre Hill for her calm and patient attitude, and for all the supportive members of the TRiO McNair Scholarship Organization. In addition, we are grateful to Mr. Doyce Dees for helping, during the decision phase of this research. The authors also wish to extend their gratitude to Mr. Andrew Tate for motivating the members of this team to get organized, during the initial portion of this project, and for helping setup the lab equipment.
References
- Centers for Disease Control and Prevention (2011) Arsine gas: Systemic agent, Atlanta, USA.
- Agency for Toxic Substances and Diseases Registry (2015) Toxic substance portal: Arsenic, USA.
- World Health Organization (2018) Arsenic, Geneva, Switzerland.
- Jain A, Raven KP, Leoppert RH (1999) Arsenite and arsenate adsorption on ferrihydrite: Surface charge reduction and net OH- release stoichiometry. Environ Sci Technol 33(8): 1179-1184.
- Healy JM, Jeffrey K (1997) Distribution of arsenic residues at the Commerce, Texas superfund site, Texas A&M University-Commerce, USA.
- Water Quality Association (2014) Arsenic fact sheet, USA.
- Hering JG, Chen PY, Wilkie JA, Elimelech M (1997) Arsenic removal from drinking water during coagulation. Journal of Environmental Engineering 123(8).
- TCEQ (2018) Hi-Yield: No further superfund environmental response actions are required on this former pesticide manufacturer in commerce, hunt county, Texas, USA.
- US EPA (2012) Basic information about arsenic in drinking water, United States environmental protection agency, USA.
- Hao L, Liu M, Wang N, Li G (2018) A critical review on arsenic removal from water using iron-based adsorbents 69: 39545-39560.
- Campbell KM, Malasarn D, Saltikov CW, Newman DK, Hering JG (2012) Simultaneous microbial reduction of iron(III) and arsenic(V) in suspensions of hydrous ferric oxide. Environ Sci Technol 40(19): 5950-5955.
- US EPA (2015) Arsenic in drinking water: Oxidation filtration iron removal, United States environmental protection agency, USA.
- Holm TR, Wilson SD (2006) Chemical oxidation for arsenic removal, Midwest technology assistance center for small public water systems.
- Clancy TM (2015) Biogeochemical evaluation of disposal options for arsenic-bearing wastes generated during drinking water treatment. The University of Michigan. Michigan, USA.
- US EPA (1996) Stabilization/solidification processes for mixed waste. Center for Remediation Technology and Tools.
- Barakat MA (2011) New trends in removing heavy metals from industrial wastewater. Arabian Journal of Chemistry 4(4): 361-377.
- Dixon J, Poli C (1995) Engineering design and design for manufacturing. A structured approach. Field Stone Publishers, Vermont, USA.
- Chen W, Parette R, Zou J, Cannon FS, Dempsey BA (2007) Arsenic removal by iron-modified activated carbon. Water Res 41(9): 1851-1858.
- Murphy NP (2016) Compost and arsenic uptake. The Effect of compost on crop arsenic uptake.
- Gustafsson O, Manukyan L, Mihranyan A (2018) High-performance virus removal filter paper for drinking water purification. Global Challenges 2(7): 1800031.
- US EPA (2002) Arsenic treatment technologies for soil, waste, and water. Solid Waste and Emergency Response, United States environmental protection agency, USA.
- Deng L, Su Y, Su H, Wang X, Zhu X (2007) Sorption and desorption of lead (II) from wastewater by green algae Cladophora fascicularis. Journal of Hazardous Materials 143(1-2): 220-225.
- Miranda PJ (2005) Gallium and arsenic/selenium recoveries from aqueous systems using silica based chelating composites. The University of Montana. Montana, USA.
- Chaudhary BK, Farrell J (2015) Understanding regeneration of arsenate-loaded ferric hydroxide-based adsorbents. Environ Eng Sci 32(4): 353-360.
- Joshi A, Chaudhuri M (1996) Removal of arsenic from ground water by iron oxide-coated sand. Journal of Environmental Engineering 122(8).
- Centers for Disease Control and Prevention (2015) Arsenic and drinking water from private wells, Atlanta, USA.
- Glastras M (1988) The precipitation of arsenic from aqueous solutions. The University of New South Wales Australia, Australia.
- Wickramasinghe SR, Han B, Zimbron J, Shen Z, Karim MN (2004) Arsenic removal by coagulation and filtration: Comparison of groundwaters from the United States and Bangladesh. Desalination 169(3): 231-244.
- Kuivenhoven M, Mason K (2020) Arsenic toxicity. Stat Pearls Publishing, USA.
- Lasat MM (2000) The use of plants for the removal of toxic metals from contaminated soil. American Association for the Advancement of Science. USA.
- Ma LQ, Komar KM, Tu C, Zhang W, Cai Y, et al. (2001) A fern that hyperaccumulates arsenic. Nature 409: 579.
- Yokoyama Y, Tanaka K, Takahashi Y (2012) Differences in the immobilization of arsenite and arsenate by calcite. Geochimica et Cosmochimica Acta 91: 202-219.
© 2021 Lucina Márcia Kuusisto. This is an open access article distributed under the terms of the Creative Commons Attribution License , which permits unrestricted use, distribution, and build upon your work non-commercially.
 a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in
a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in 







.jpg)






























 Editorial Board Registrations
Editorial Board Registrations Submit your Article
Submit your Article Refer a Friend
Refer a Friend Advertise With Us
Advertise With Us
.jpg)






.jpg)














.bmp)
.jpg)
.png)
.jpg)










.jpg)






.png)

.png)



.png)






